Abstract
Background
Multiple gastric cancers at the same time (synchronous) or recurrence after 1 year (metachronous) are frequently encountered. Since their genetic profiles were not well elucidated, we molecularly subtyped the genetic events of synchronous and metachronous early-stage gastric cancers.
Methods
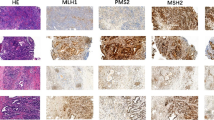
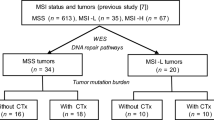
We studied mismatch repair (MMR) genes in 84 tumors from 31 patients (15 synchronous and 16 metachronous) by immunohistochemistry. We performed microsatellite instability analysis and targeted sequencing of 58 significantly mutated genes (SMGs) in 35 tumors from thirteen patients. Genomic data from TCGA were used for comparisons with advanced-stage cancers.
Results
Among the 31 patients, at least one deficient-MMR (dMMR) tumor was observed in eight (26%). Of eight patients, seven showed a mixture of proficient-MMR (pMMR) and dMMR tumors. The one case with only dMMR had six recurrent tumors within 2 years. To further subtype, we sequenced 58 SMGs in 35 samples (25 pMMR and 10 dMMR) from thirteen patients. In 35 samples, 163 mutations were identified, but none matched in almost cases, strongly indicating different clonal origins, whether synchronous or metachronous occurrences. Of the 25 pMMR cases, 1 belonged to Epstein–Barr virus (EBV), 24 belonged to chromosomal instability (CIN) subtypes. Of the thirteen cases, repetitive CIN, a mixture of CIN and MSI, a mixture of CIN and EBV, and repetitive MSI were observed in nine (70%), two (15%), one (8%) and one (8%), respectively.
Conclusions
Despite multiple tumors occurring in the same patient simultaneously or several years apart, clonal origin was totally different. ‘Switching’ or ‘mixing’ of dMMR and pMMR, EBV or CIN occurred, which had clinical relevance with regard to immunotherapy.






Similar content being viewed by others
Abbreviations
- MMR:
-
Mismatch repair
- SMGs:
-
Significantly mutated genes
- dMMR:
-
Deficient-MMR
- pMMR:
-
Proficient-MMR
- EBV:
-
Epstein–Barr virus
- EBER:
-
Epstein–Barr virus encoded small mRNA’s
- MSI:
-
Microsatellite instability
- CIN:
-
Chromosomal instability
- ESD:
-
Endoscopic submucosal dissection
- TCGA:
-
The Cancer Genome Atlas
- ICGC:
-
International Cancer Genome Consortium
- IHC:
-
Immunohistochemistry
- FFPE:
-
Formalin-fixed paraffin-embedded
- COSMIC:
-
Catalog of Somatic Mutations in Cancer
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Hasuo T, Semba S, Li D, et al. Assessment of microsatellite instability status for the prediction of metachronous recurrence after initial endoscopic submucosal dissection for early gastric cancer. Br J Cancer. 2007;96(1):89–94.
Lim JH, Kim SG, Choi J, et al. Risk factors for synchronous or metachronous tumor development after endoscopic resection of gastric neoplasms. Gastric Cancer. 2015;18(4):817–23.
Boda T, Ito M, Oka S, et al. Characteristics of metachronous gastric tumors after endoscopic submucosal dissection for gastric intraepithelial neoplasms. Gastroenterol Res Pract. 2014;2014:863595.
Kato M, Nishida T, Yamamoto K, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62(10):1425–32.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9.
Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48(2):225–9.
Nakagomi T, Goto T, Hirotsu Y, et al. New therapeutic targets for pulmonary sarcomatoid carcinomas based on their genomic and phylogenetic profiles. Oncotarget. 2018;9(12):10635–49.
Hirotsu Y, Nakagomi H, Sakamoto I, et al. Multigene panel analysis identified germline mutations of DNA repair genes in breast and ovarian cancer. Mol Genet Genomic Med. 2015;3(5):459–66.
Capel E, Flejou JF, Hamelin R. Assessment of MLH1 promoter methylation in relation to gene expression requires specific analysis. Oncogene. 2007;26(54):7596–600.
Iijima Y, Hirotsu Y, Amemiya K, et al. Very early response of circulating tumour-derived DNA in plasma predicts efficacy of nivolumab treatment in patients with non-small cell lung cancer. Eur J Cancer. 2017;86:349–57.
Hirotsu Y, Kojima Y, Okimoto K, et al. Comparison between two amplicon-based sequencing panels of different scales in the detection of somatic mutations associated with gastric cancer. BMC Genomics. 2016;17(1):833.
Amemiya K, Hirotsu Y, Goto T, et al. Touch imprint cytology with massively parallel sequencing (TIC-seq): a simple and rapid method to snapshot genetic alterations in tumors. Cancer Med. 2016;5(12):3426–36.
Hirotsu Y, Nakagomi H, Sakamoto I, et al. Detection of BRCA1 and BRCA2 germline mutations in Japanese population using next-generation sequencing. Mol Genet Genomic Med. 2015;3(2):121–9.
Wang K, Kan J, Yuen ST, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43(12):1219–23.
Kakiuchi M, Nishizawa T, Ueda H, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46(6):583–7.
Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46(6):573–82.
Wong SS, Kim KM, Ting JC, et al. Genomic landscape and genetic heterogeneity in gastric adenocarcinoma revealed by whole-genome sequencing. Nat Commun. 2014;5:5477.
Zang ZJ, Cutcutache I, Poon SL, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44(5):570–4.
Goto T, Hirotsu Y, Mochizuki H, et al. Mutational analysis of multiple lung cancers: discrimination between primary and metastatic lung cancers by genomic profile. Oncotarget. 2017;8:31133–43.
Liu Y, Sethi NS, Hinoue T, et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell. 2018;33(4):721–735e8.
Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392–7.
Mori G, Nakajima T, Asada K, et al. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: results of a large-scale, multicenter cohort study in Japan. Gastric Cancer. 2016;19(3):911–8.
Kim TM, Jung SH, Kim MS, et al. The mutational burdens and evolutionary ages of early gastric cancers are comparable to those of advanced gastric cancers. J Pathol. 2014;234(3):365–74.
Goto T, Hirotsu Y, Nakagomi T, et al. Detection of tumor-derived DNA dispersed in the airway improves the diagnostic accuracy of bronchoscopy for lung cancer. Oncotarget. 2017;8:79404–13.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–1.
Le Dung T, Durham Jennifer N, Smith Kellie N, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13.
Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Maekita T, Nakazawa K, Mihara M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12(3 Pt 1):989–95.
Nakajima T, Maekita T, Oda I, et al. Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epidemiol Biomark Prev. 2006;15(11):2317–21.
Minoru T, Nita A, Hiromu S, et al. Aberrant Methylation in Gastric Cancer Associated with the CpG island methylator phenotype. Cancer Res. 1999;59(21):5438–42.
Suet Yi L, Siu Tsan Y, Lap Ping C, et al. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999;59(1):159–64.
Min BH, Hwang J, Kim NK, et al. Dysregulated Wnt signalling and recurrent mutations of the tumour suppressor RNF43 in early gastric carcinogenesis. J Pathol. 2016;240(3):304–14.
Yashiro M, Hirakawa K, Boland CR. Mutations in TGFbeta-RII and BAX mediate tumor progression in the later stages of colorectal cancer with microsatellite instability. BMC Cancer. 2010;10:303.
Acknowledgements
This study was supported by a Grant-in-Aid for Genome Research Project from Yamanashi Prefecture (to M.O. and Y.H.), The Japan Society for the Promotion of Science (JSPS) KAKENHI Early-Career Scientists (Grant Number JP18K16292 to Y.H.), Research Grant for Young Scholars (to Y.H.), The YASUDA Medical Foundation (to Y.H.) and The Uehara Memorial Foundation (to Y.H.). We thank all medical and ancillary hospital staff and the patients for consenting to participate. We also thank Kanoko Sano, Saki Hiraga, Shunsuke Watanabe, and Eri Ishii for technical support. Finally, we thank James P. Mahaffey, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2019_1547_MOESM2_ESM.pdf
Supplemental Figure 1. Relationship between mutated genes and variant allele fractions. Average of variant allele fractions and the number of mutations were plotted (PDF 9 kb)
535_2019_1547_MOESM3_ESM.pdf
Epstein–Barr virus (EBV) infection status was determined by in situ hybridization targeting EBV encoded small mRNA’s (EBER). Representative images for hematoxylin-eosin staining and EBER in situ hybridization performed in EBV-positive (T1 in Case #3) and EBV-negative (T1 in Case #16) gastric cancer tissues. Scale bar: 100 μm (PDF 205 kb)
Rights and permissions
About this article
Cite this article
Takaoka, S., Hirotsu, Y., Ohyama, H. et al. Molecular subtype switching in early-stage gastric cancers with multiple occurrences. J Gastroenterol 54, 674–686 (2019). https://doi.org/10.1007/s00535-019-01547-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01547-z