Abstract
Background
HNF1B gene mutations are an important cause of bilateral (cystic) dysplasia in children, complicated by chronic renal insufficiency. The clinical variability, the absence of genotype-phenotype correlations, and limited long-term data render counseling of affected families difficult.
Methods
Longitudinal data of 62 children probands with genetically proven HNF1B nephropathy was obtained in a multicenter approach. Genetic family cascade screening was performed in 30/62 cases.
Results
Eighty-seven percent of patients had bilateral dysplasia, 74% visible bilateral, and 16% unilateral renal cysts at the end of observation. Cyst development was non-progressive in 72% with a mean glomerular filtration rate (GFR) loss of − 0.33 ml/min/1.73m2 per year (± 8.9). In patients with an increase in cyst number, the annual GFR reduction was − 2.8 ml/min/1.73m2 (± 13.2), in the total cohort − 1.0 ml/min/1.73m2 (±10.3). A subset of HNF1B patients differs from this group and develops end stage renal disease (ESRD) at very early ages < 2 years. Hyperuricemia (37%) was a frequent finding at young age (median 1 year), whereas hypomagnesemia (24%), elevated liver enzymes (21%), and hyperglycemia (8%) showed an increased incidence in the teenaged child. Genetic analysis revealed no genotype-phenotype correlations but a significant parent-of-origin effect with a preponderance of 81% of maternal inheritance in dominant cases.
Conclusions
In most children, HNF1B nephropathy has a non-progressive course of cyst development and a slow-progressive course of kidney function. A subgroup of patients developed ESRD at very young age < 2 years requiring special medical attention. The parent-of-origin effect suggests an influence of epigenetic modifiers in HNF1B disease.
Similar content being viewed by others
Introduction
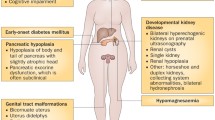
Congenital anomalies of the kidneys including renal dysplasia are a common cause of renal failure in children [1]. Diagnosis of kidney dysplasia in clinical routine is based on ultrasonography, showing an echo-bright kidney with or without cysts and poor corticomedullary differentiation [2]. Renal histology defines kidney dysplasia by showing incompletely branched ducts surrounded by undifferentiated and metaplastic stroma [3, 4]. In a significant number of cases, gene mutations in HNF1B on chromosome 17q21.3 have been identified as underlying cause. HNF1B encodes for hepatocyte nuclear factor-1ß (HNF-1ß), a transcription factor that is expressed early in embryonic development. HNF-1ß acts as a homo- or heterodimer with HNF-1α and plays a key role for tissue-specific regulations of gene expression in various organs such as the kidneys, liver, biliary ducts, pancreas, and the urogenital tract [5, 6]. First, HNF1B gene mutations were identified in patients with maturity onset diabetes of the young (MODY 5) in 1997 [7]. Today, HNF1B disease is recognized to represent an autosomal dominant syndromal disorder comprising renal cystic disease, diabetes mellitus, elevated liver enzymes, hyperuricemia, and pancreatic and genital tract malformations (MIM 137920; renal cysts and diabetes syndrome, RCAD) [8]. Some patients with HNF1B mutations show hypomagnesemia caused by hypermagnesiuria [9] which is in part due to the control of FXYD2 expression by HNF-1ß in the distal tubule [10]. HNF1B mutations are also identified in pediatric patients with isolated renal dysplasia with cysts [11,12,13,14]. The nephropathy in these cases is part of the autosomal dominant tubulointerstitial kidney disease spectrum ADTKD [15], formerly known as medullary cystic kidney disease (MCKD). Next to HNF1B three other genes (MUC1, UMOD, and REN) with shared clinical features have been assigned to this prototypic group of nephropathies. We here focussed on genetically proven HNF1B nephropathy in children and sought to define the longitudinal clinical course, the progression of renal disease, and the manifestation of extrarenal symptoms. For this purpose, we initiated a national clinical registry encompassing a pediatric cohort of young age. Additional data from the Czech Republic and Poland were included in a collaborative approach.
Material and methods
Patients
Sixty-two children probands (39 males; aged 0–18 years) treated at one of 11 pediatric nephrology units in Germany, the Czech Republic, and Poland, were recruited, and annual data were collected from a total of 323 questionnaires (median follow-up 4 years; range 1 month–19 years, Suppl. Material 1a, b). Clinical and laboratory data as well as repeated renal ultrasound data were collected as described below. All patients entered in the study had primarily been seen by expert pediatric nephrologists. A renal phenotype with cystic or non-cystic kidney dysplasia and/or tubulointerstitial kidney disease (TKD) led to the initiation of HNF1B mutational analysis. Inclusion criterion of the study was confirmed HNF1B nephropathy with proven mutation in HNF1B. For this reason, the HNF1B score published by Faguer et al. [16] to identify patients suitable for HNF1B mutation testing was not applicable in the present study.
Mutation analysis
Genomic deoxyribonucleic acid (DNA) was extracted from peripheral blood. Exons 1–9 of HNF1B, including intron-exon boundaries, were amplified by polymerase chain reaction (PCR) and subjected to exon-specific next-generation sequencing (NGS) and Sanger sequencing. The result was compared to the published sequence NM_000458.2. Multiplex ligation-dependent probe amplification (MLPA) analysis was performed to determine the presence of copy number variations, deletions, or duplications (SALSA MLPA P241 probe mix; MRC-Holland, Amsterdam, Netherlands) [17], including GCK, HNF1A, HNF1B, and HNF4A.
Renal ultrasound
Repeated renal ultrasound examinations were performed annually at each center. Renal dysplasia was defined as poor corticomedullary differentiation, echo-bright kidneys, or both [2]. For medical and ethical reasons, affected children are generally not biopsied which might be a limitation of the study. Renal cysts were categorized by number (n ≤ 5 or n > 5), and a cyst score was applied to define an increase in cyst number over time (no cysts, score 0; ≤ 5 cysts, score 1; > 5 cysts, score 2). Progressive disease was defined as cyst progression in at least one kidney.
Clinical and laboratory data
Baseline and follow-up data of age, height, onset of nephropathy, ESRD, hypomagnesemia, hyperglycemia, hyperuricemia, elevated liver enzyme activity, exocrine pancreas insufficiency, and anomalies of the genital tract were documented following external physical and ultrasound examination and laboratory assessment. Age-dependent reference limits were applied [18,19,20]. Hypomagnesemia was defined as repeated serum magnesium concentrations lower than 0.65 mmol/l. The estimated glomerular filtration rate (eGFR) was calculated from serum creatinine and body height according to the Schwartz formula [21]. The CKD Work Group’s KDIGO guidelines were used to define chronic kidney disease (CKD) stages I–V [22].
Family histories were documented including genetic results and reported HNF1B-related symptoms as provided by the cooperating centers.
Statistical analysis
Data are given as the median and/or mean with standard deviation (SD). Statistical analysis was assessed using Student’s t test. Statistical significance was defined as p ≤ 0.05.
Results
Genetics
HNF1B whole gene deletions (exons 1–9) were detected in 33/62 patients (53%), missense mutations in 11 (18%), nonsense (stop) mutations in 7 (11%), and splice mutations/gross insertions in 11 (18%) (Table 1). Parental DNA of 28 families (30 patients) was subjected to cascade screening, and parental transmission of HNF1B gene mutations was observed in 16/28 families with maternal transmission in 13/16 (81%) and paternal in 3/16 (19%). 11/16 (71%) parental mutation carriers exhibited symptoms related to HNF1B. Four parental mutation carriers had no documented clinical symptoms. We have no information whether a systematic screening in these four individuals was performed which is a limitation of this study. In one parental mutation carrier with proven genetic result, no clinical information was available. De novo mutations were detected in 12/30 patients (40%). Among these, 10/12 (83%) constitute complete gene deletions, 1/12 a missense, and 1/12 a stop mutation. Three missense mutations (Asp82Asn, Val61Gly, and Gly76Cys) are annotated in the gnomAD database with allele frequencies of approximately 1–2:1000 in large populations of different ethnic origins (http://gnomad.broadinstitute.org); however, the phenotype of the study patient affected by Asp82Asn shows typical HNF1B disease, and both Val61Gly and Gly76Cys have been reported in different HNF1B studies in patients with HNF1B disease (http://www.hgmd.cf.ac.uk). As CAKUT is as frequent as 1% in the general population, these missense mutations most likely represent hypomorphic variants associated with HNF1B disease in conjunction with other genetic or environmental events [12, 23]. The same holds true for patient 10, offspring of consanguineous union and affected by a homozygous Arg232Cys mutation, and a full-blown picture of HNF1B disease.
There were no statistically significant differences in renal function or phenotypes among carriers of different HNF1B mutation types (p > 0.05 between all groups; Table 2 and Suppl. Table 1). The mean GFR at the end of observation in the group of patients with deletions was 106.8 ml/min/1.73m2 (mean age 12.0 years, median age 8.0 years) vs. 98.0 ml/min/1.73m2 (mean age 8.4 years, median age 7.0 years) in the group with non-deletion mutations (p > 0.05).
Renal phenotype
Prenatal dysplasia was documented for 32/62 patients (52%); (no data n = 30). At the end of follow-up, ultrasound revealed bilateral dysplasia in 54 patients (87%), unilateral dysplasia in 3, unilateral dysplasia with contralateral agenesis in 4, and no documented dysplasia in 1 (Fig. 1a).
a Prevalence of dysplasia and cysts in patients with HNF1B mutation at last follow-up. b Cyst score over time (n = 94 kidneys from 47 patients with at least two renal ultrasound examinations). In total, 23/94 (24%) kidneys showed a progress. Patients defined as progressive with respect to renal cysts showed a progress in at least one or both kidneys. No progress n = 34/47 patients (72%); progress n = 13/47 patients (28%). We defined a progress in renal cysts, when there was a progress in at least one of the patients’ kidneys
Bilateral renal cysts—at the end of follow-up—were present in 46/62 patients (74%), unilateral cysts in 10 (16%), and no cysts in 6 (10%). Most patients experienced no statistically significant increase (p > 0.05) in the number of renal cysts at a median follow-up of 4 years (mean 5.2 years; range 1 month–19 years). Of the total cohort with at least two renal ultrasound examinations, no increase was documented in 34/47 patients (72%); and an increase in 13/47 patients (28%): in 4 patients, cysts were observed for the first time during the course of the study; and in nine patients, the cyst score increased from 1 to 2; Fig. 1b).
At the end of follow-up, bilateral dysplasia with cysts was found in 46/62 patients (74%), and unilateral dysplasia with cysts and contralateral kidney agenesis was found in two. Unilateral cystic dysplasia with a normal contralateral kidney was documented for eight patients (13%). From the remaining six patients, five showed renal dysplasia but no cysts.
Renal function
Kidney function exhibited wide interindividual variability. At the end of follow-up, ESRD was documented in 5/62 (8%) with a median age of 15 months (range 3 months–6 years); all had undergone transplantation. The median time from initial diagnosis to ESRD was 7 months (range 1 month–4 years).
At the end of follow-up, CKD I was documented in 36/62 patients (58%), CKD II in 15 (24%), CKD III in 2 (3%), CKD IV in 4 (7%), and CKD V in 5 patients (8%); the median age of study patients was 8 years (range 0–19 years; Fig. 2a).
a Chronic kidney disease (CKD) of the total cohort at the end of the observation (n = 62, mean 8.2 years, median 8 years, range 0–19 years). b Chronic kidney disease (CKD) at first description. Included patients > 2 years to avoid misinterpretation of early creatinine values with at least two follow-ups (n = 33, mean 6.3 years, median 4 years, range 2–18 years). c Chronic kidney disease (CKD) at last description. Included patients > 2 years to avoid misinterpretation of early creatinine values with at least two follow-ups (n = 33, mean 11.2 years, median 12 years, range 7–18 years)
To calculate the annual reduction in GFR loss, we only included data from patients > 2 years of age—to avoid misinterpretation of early creatinine values—with at least two documented GFR-values. In the total cohort, the mean annual reduction in GFR was − 1.0 ml/min/1.73 m2 (± 10.3). For the group of patients with an increase in the number of cysts, the mean annual reduction in GFR was − 2.8 ml/min/1.73m2/year (± 13.2) whereas for the group with no increase in cyst number, the annual GFR development was − 0.33 ml/min/1.73m2 (± 9.5).
At study onset, we documented for these patients CKD I in 61% (20/33), CKD II in 27% (9/33), CKD III in 3% (1/33), CKD IV in 9% (3/33), and CKD V in no patient (median age 4 years, range 2–18 years; Fig. 2b).
At the end of follow-up in these 33 patients, we documented: CKD I in 64% (21/33), CKD II in 24% (8/33), CKD III in no patient, CKD IV in 9% (3/33), and CKD V in 3% (1/33) (median age 12 years, range 5–19 years; Fig. 2c).
Biochemical data
Hypomagnesemia (Table 3) was detected in 12/50 children (24%) at a median age of 10 years (mean 8.9 years; range 0–16 years). Magnesium values ranged from 0.5 to 0.6 mmol/l (mean 0.55 mmol/l). 7/29 (24%) patients with CKD I presented with hypomagnesemia (median age 12; mean 10; range 0–16 years). In 6/7 patients, hypomagnesemia was diagnosed at the same time as HNF1B nephropathy, in one patient 8 years after the initial diagnosis.
Hyperuricemia was detected in 19/52 patients (37%) at a median age of 1 year (range 0–17 years) and already in 11/32 patients (34%) with CKD I. For all patients, hyperuricemia was diagnosed at the same time as HNF1B nephropathy. Uric acid concentrations ranged from 363 to 500 μmol/l (mean 429 μmol/l). No clinical symptoms of gout were present.
Elevated liver enzyme activity was diagnosed in 12/58 patients (21%) at a median age of 11 years (mean 10.4; range 0–18 years). For eight of these patients, elevated liver enzymes were documented at the same time as HNF1B nephropathy at a median age of 14 years, for four patients with a median lag time of 10.5 years after initial manifestation of HNF1B nephropathy.
4/50 patients (8%) experienced recurrent hyperglycemia, elevated HbA1c levels, or both. The age at manifestation was 4, 12, and 14 years, respectively, and in one patient, a neonatal diabetes mellitus (DM) with inhomogeneous pancreas texture was diagnosed shortly after birth.
Urogenital abnormalities
One of the 62 patients had hypoplastic testicles; no other urogenital abnormalities were documented.
Discussion
The clinical differential diagnosis of HNF1B nephropathy includes other genetic forms of congenital anomalies of the kidneys and urinary tract (CAKUT), ADTKD-UMOD, -MUC1, or -REN but also autosomal dominant polycystic kidney disease (ADPKD), depending on the ultrasound picture and extrarenal symptoms. In exceptional cases, ADPKD can manifest itself in very young patients (very early onset [VEO]-ADPKD) [24]. In most adult cases, the prognosis of ADPKD is unfavorable, with consistent growth of cysts and deterioration of renal function. The progression of cyst growth and renal insufficiency in HNF1B nephropathy has not been as thoroughly studied so far and was central focus of the here presented study.
Genotype-phenotype correlation
The present work shows a lack of genotype-phenotype correlations among the various types of HNF1B gene mutations, as has been reported previously [11, 14]. One could have expected gene deletions and loss-of-function mutations with higher frequency in patients with more severe disease as has been reported for other kidney disorders [25, 26]. However, this issue is controversially discussed, and other authors even describe a more favorable renal outcome in patients with gene deletions compared to other mutations in HNF1B [27,28,29]. Possible explanations include a dominant negative effect of HNF1B non-deletion mutations or the involvement of other genes located in the deletion interval on chromosome 17q12.3. This interval spans additional 14 genes to HNF1B and affected patients are also at risk to develop neuropsychological symptoms. These comprise intellectual disabilities, learning difficulties, externalization disorders as attention-deficit hyperactivity disorder and autistic traits with variable severity [30,31,32]. Few patients with intellectual disability and intragenic HNF1B mutations have also been described [32]. To date, however, no studies have systematically called for expert neuropsychological testing in HNF1B patients, such as those included in the present study. Follow-up studies will have to address this important issue strongly impacting the quality of life of affected individuals.
Like in previous studies, HNF1B mutations were equally distributed among boys and girls. De novo mutations were observed in 40% of patients with a preponderance of deletion mutations (83%). A similar observation was made in [14]. Paired segmental duplications along with breakpoints are most likely involved in the pathogenesis of this recurrent chromosomal microdeletion [33]. One striking observation of the present study is the high degree (81%) to which HNF1B mutations are inherited through the maternal lineage in familial cases. Ulinski et al. reported dominant inheritance in 8/17 families; however, no parental data were presented [11]. In a study involving 42 patients with complete gene deletions, Heidet et al. identified de novo mutations in 14 of these patients; for seven patients, there was proven dominant inheritance, with maternal transmission in 6/7 (86%) [14]. A similar parent-of-origin effect was recently described for non-renal autosomal disease, e.g., familial early puberty [34] and hereditary paraganglioma [35]. Alterations of genomic imprinting or maternal imprinting of modifying genes were believed to be involved in these cases. No cascade testing after routine scans in pregnant women has been performed in our study ruling out a selection bias. Alternatively, the fertility of adult men with HNF1B mutations may be reduced. In-depth genetic studies will be necessary to elucidate these parent-of-origin mechanisms.
Renal phenotype
Ninety-eight percent of the study patients showed renal dysplasia and 90% renal cysts. At a median follow-up of 4 years, there was no significant increase in the number of renal cysts in 72% of patients. The mean annual decrease in GFR was − 1.0 ml/min/1.73m2. These findings indicate that HNF1B nephropathy in children is a rather slow-progressive disorder with respect to cyst development and to loss of kidney function. These characteristics distinguish the HNF1B phenotype from the polycystic kidney disease (PKD) spectrum.
Overall, the detection rate of renal cysts in patients with HNF1B mutations is comparable across the published studies performed by pediatric nephrologists (84% in [11], 94% in [13]). In addition, both isolated bilateral hyperechogenic kidneys and cystic dysplasia in the antenatal period can be highly indicative of HNF1B nephropathy [13, 14], and a postnatal diagnostic clarification is strongly advised for these newborns including a renal ultrasound in their parents.
Renal function
Among a subset of HNF1B patients, CRF and ESRD seem to develop when the patients are very young. Five patients rapidly developed ESRD with a GFR below 30 ml/min1.73m2 already at an early age. Extrarenal symptoms did not differ from the rest of the cohort but all were affected by severe bilateral dysplasia. ESRD is a sequela of severe dysplasia of both kidneys, independent of the underlying gene mutation. Presumably, additional genetic factors or modifiers, environmental factors, or epigenetic influences aggravate the disease in this subset of patients. Similar observations have been made in the subset of young children with VEO-ADPKD [36], for whom additional genetic variants were identified in PKHD1 or HNF1B and were presumed to act as disease modifiers. VEO-HNF1B nephropathy requires special attention and medical care, including early renal replacement therapy.
Non-VEO-HNF1B nephropathy has a more favorable course, with only a slow decline of renal function over time during childhood; normal renal function is preserved for many children. In children with cyst progression, the annual decline in GFR seems to be pronounced. In previous studies, a normal GFR (70 ml/min/1.73m2 or higher) was documented in 56% of pediatric study patients in [11] and in 39% of pediatric study patients in [13]. During adulthood, loss of renal function seems to accelerate, as suggested by the results of Faguer et al. in a series of 27 adult HNF1B patients with a median annual decrease in GFR of − 2.45 ml/min/1.73m2 [37]. Continuous follow-up of the pediatric registry with transition into adulthood will be important for defining this acceleration of decline in renal function as patients’ age increases. Possible causes might be related to arterial hypertension, DM, proteinuria, or dietary salt intake.
Extrarenal manifestations
Hypomagnesemia was diagnosed in 24% of our study patients but higher rates have been reported in cohorts composed mostly of older patients. Renal magnesium wasting in HNF1B disease was first described in [9] in 44% of 21 patients. Faguer et al. reported hypomagnesemia (Mg < 0.75 mmol/l) in 63% of patients with a median age of 35 years at last follow-up [37]. In a case series of three adult male patients, pronounced hypomagnesemia was the first clinical manifestation of ADTKD-HNF1B [38]. Besides significant differences in defining hypomagnesemia, the development of this condition seems to be strongly age-related.
Hyperuricemia was documented in 37% of our study patients, with an early onset at a median patient age of 1 year. Only 20% of patients in the study by Bingham et al. [39] exhibited elevated uric acid levels. Because uric acid concentrations are not routinely measured at many pediatric centers, hyperuricemia may be underdiagnosed. Among patients with kidney dysplasia, hyperuricemia disproportionate to renal function highly suggests HNF1B nephropathy as underlying cause. However, an important differential diagnosis is ADTKD caused by variants in uromodulin (ADTKD-UMOD), presenting as familial juvenile hyperuricemic nephropathy and MCKD [40, 41]. The cause of hyperuricemia in both ADTKD-UMOD and ADTKD-HNF1B is not well understood, but hyperuricemia seems to be a consequence of tubulointerstitial dysfunction. Novel experimental studies have linked HNF1B to mitochondrial energy metabolism in renal tubular cells [42] providing a possible link to transcellular substrate movements. More experimental work will be necessary for better defining the role of HNF1B in uric acid transport and metabolism.
Hyperglycemia, elevated HbA1c levels, or both were found in only 8% of our patients. Decramer et al. [13] found DM in 17% of patients; Bingham et al. [39] in 58% (mean age at diagnosis 25 years); Faguer et al. [37] in 48% (median age 35 years); and Edghill et al. [43] in 48%. These results indicate that impaired glucose tolerance and DM are rarely observed in childhood but, even more so than hypomagnesemia, develop later in the clinical course of HNF1B disease. However, under certain circumstances, e.g., after renal transplantation with concomitant high doses of steroids, tacrolimus, or both, DM may be unmasked in HNF1B-positive patients [44], requiring specific treatment and often a change in the immunosuppressive regimen. New-onset diabetes after transplant (NODAT) is a serious complication compromising renal graft function [45]. Therefore, screening for HNF1B mutations should be considered pre-transplant for patients with ESRD caused by (cystic) kidney dysplasia. In a subgroup of patients, HNF1B disease first manifests as a disturbance of glucose metabolism [46]. A recent Norwegian study estimated that the overall prevalence of monogenic diabetes gene mutations (including HNF1A, HNF4A, HNF1B, GCK, and INS) in children with autoantibody-negative DM is very rare with 6.5% [47]. A study using high-throughput genetic analysis of 4016 patients with type 2 DM (34% with age at diagnosis of < 40 years) identified only one HNF1B mutation (age at diagnosis 14 years), whereas mutations in HNF1A and GCK occurred much more frequently [48]. HNF1B seems to play a minor role in children (and adults) with isolated autoantibody-negative DM.
Elevated liver enzyme activity was detected in 21% of our patients, in 13% in [39], and in 40% in [37]; thus, there is a high degree of variability among the separate studies. Results of liver ultrasound seem to be normal in most cases reported so far; however, a longitudinal follow-up of our pediatric patients into adulthood will be of interest in elucidating the long-term effects of liver dysfunction in HNF1B disease.
Genital tract anomalies resulting from Müllerian duct aplasia and failure of fusion of the Müllerian ducts have repeatedly been described in patients with HNF1B mutations [49, 50]. In our cohort, only one patient was found to have hypoplastic testicles and no other urogenital abnormalities were detected. Bingham et al. [39] described uterine malformations in 14% of female patients and genital tract malformations in 5% of male patients. Edghill et al. [43] found genital tract anomalies in 9% of patients. Oram et al. identified mutations in HNF1B in 18% (9/50) of women with combined uterine and renal abnormalities but in none (0/58) with isolated malformations of the uterus [51]. Overall, genital tract malformations are an inconsistent finding. However, when they are combined with renal abnormalities, HNF1B mutation analysis seems advised.
In summary, this report presents the results of a longitudinal dataset from one of the largest pediatric cohorts of patients with HNF1B nephropathy. With few exceptions, HNF1B nephropathy among children is a rather non-progressive disorder with respect to cyst development and a slowly progressive disease with respect to kidney damage. In contrast, VEO-HNF1B nephropathy is associated with high morbidity rates and early ESRD. Hyperuricemia is a frequent finding in very young patients, whereas the prevalence of hypomagnesemia, elevated liver enzyme activity, and hyperglycemia is higher among teenagers. A close genotype-phenotype correlation is lacking; however, we describe a significant parent-of-origin effect in HNF1B disease, with an 80% preponderance of maternal inheritance. Future studies employing the combined efforts of international registries are necessary for identifying the underlying mechanisms.
References
Lewis MA, Shaw J, Sinha MD, Adalat S, Hussain F, Castledine C, van Schalkwyk D, Inward C (2010) UK Renal Registry 12th Annual Report (December 2009): Chapter 14: Demography of the UK paediatric renal replacement therapy population in 2008. Nephron Clin Pract 115:c279–c288
Vester U, Kranz B, Hoyer PF (2010) The diagnostic value of ultrasound in cystic kidney diseases. Pediatr Nephrol 25(2):231–240
Risdon RA (1971) Renal dysplasia. I. Clinico-pathological study of 76 cases. J Clin Pathol 24:57–71
Kakkar N, Menon S, Radotra BD (2006) Histomorphology of renal dysplasia – an autopsy study. Fetal Pediatr Pathol 25(2):73–86
Ott MO, Rey-Campos J, Cereghini S, Yaniv M (1991) vHNF1 is expressed in epithelial cells of distinct embryonic origin during development and precedes HNF1 expression. Mech Dev 36(1–2):47–58
Coffinier C, Barra J, Babinet C, Yaniv M (1999) Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev 89(1–2):211–213
Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI (1997) Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY [Letter]. Nat Genet 17(4):384–385
Lindner TH, Njolstad PR, Horikawa Y, Bostad L, Bell GI, Sovik O (1999) A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum Mol Genet 8(11):2001–2008
Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, Hennekam RC, Ledermann SE, Rees L, van't Hoff W, Marks SD, Trompeter RS, Tullus K, Winyard PJ, Cansick J, Mushtaq I, Dhillon HK, Bingham C, Edghill EL, Shroff R, Stanescu H, Ryffel GU, Ellard S, Bockenhauer D (2009) HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 20(5):1123–1131
Ferrè S, Veenstra GJ, Bouwmeester R, Hoenderop JG, Bindels RJ (2011) HNF-1B specifically regulates the transcription of the γa-subunit of the Na+/K+-ATPase. Biochem Biophys Res Commun 404(1):284–290
Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, Clauin S, Deschênes G, Bouissou F, Bensman A, Bellanné-Chantelot C (2006) Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol 17(2):497–503
Weber S, Moriniere V, Knüppel T, Charbit M, Dusek J, Ghiggeri GM, Jankauskiené A, Mir S, Montini G, Peco-Antic A, Wühl E, Zurowska AM, Mehls O, Antignac C, Schaefer F, Salomon R (2006) Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol 17(10):2864–2870
Decramer S, Parant O, Beaufils S, Clauin S, Guillou C, Kessler S, Aziza J, Bandin F, Schanstra JP, Bellanné-Chantelot C (2007) Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol 18(3):923–933
Heidet L, Decramer S, Pawtowski A, Morinière V, Bandin F, Knebelmann B, Lebre AS, Faguer S, Guigonis V, Antignac C, Salomon R (2010) Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol 5(6):1079–1090
Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O (2015) Kidney disease: improving global outcomes: autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--a KDIGO consensus report. Kidney Int 88(4):676–683
Faguer S, Chassaing N, Bandin F, Prouheze C, Garnier A, Casemayou A, Huart A, Schanstra JP, Calvas P, Decramer S, Chauveau D (2014) The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int 86(5):1007–1015
Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G (2002) Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 30(12):e57
Fischbach KF, Zawta B (1992) Age-dependent reference limits of several enzymes in plasma at different measuring temperatures. Klin Lab 38:555–561
Meites S (1989) Pediatric clinical chemistry. American Association for Clinical Chemistry, Washington DC
Witt I, Trendelenburg C (1982) Joint study to establish reference values for clinical chemical parameters in childhood. J Clin Chem Clin Biochem 20(4):235–242
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Am 34(3):571–590
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013) Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3(1):1–150
Nicolaou N, Renkema KY, Bongers EM, Giles RH, Knoers NV (2015) Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol 11(12):720–731
Bergmann C (2015) ARPKD and early manifestations of ADPKD: the original polycystic kidney disease and phenocopies. Pediatr Nephrol 30(1):15–30
Seys E, Andrini O, Keck M, Mansour-Hendili L, Courand PY, Simian C, Deschenes G, Kwon T, Bertholet-Thomas A, Bobrie G, Borde JS, Bourdat-Michel G, Decramer S, Cailliez M, Krug P, Cozette P, Delbet JD, Dubourg L, Chaveau D, Fila M, Jourde-Chiche N, Knebelmann B, Lavocat MP, Lemoine S, Djeddi D, Llanas B, Louillet F, Merieau E, Mileva M, Mota-Vieira L, Mousson C, Nobili F, Novo R, Roussey-Kesler G, Vrillon I, Walsh SB, Teulon J, Blanchard A, Vargas-Poussou R (2017) Clinical and genetic spectrum of Bartter syndrome type 3. J Am Soc Nephrol 28(8):2540–2552
Weber S, Gribouval O, Esquivel EL, Morinière V, Tête MJ, Legendre C, Niaudet P, Antignac C (2004) NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int 66(2):571–579
Dubois-Laforgue D, Cornu E, Saint-Martin C, Coste J, Bellanné-Chantelot C, Timsit J (2017) Monogenic diabetes study group of the Société Francophone du Diabète. Diabetes, associated clinical spectrum, long-term prognosis, and genotype/phenotype correlations in 201 adult patients with hepatocyte nuclear factor 1B (HNF1B) molecular defects. Diabetes Care 40(11):1436–1443
Clissold RL, Harries LW, Ellard S, Bingham C, Hattersley AT (2018) Comment on Dubois-Laforgue et al. Diabetes, associated clinical spectrum, long-term prognosis, and genotype/phenotype correlations in 201 adult patients with hepatocyte nuclear factor 1B (HNF1B) molecular defects. Diabetes Care 2017; 40:1436–1443. Diabetes Care 41(1):e7. https://doi.org/10.2337/dc17-1672
Dubois-Laforgue D, Cornu E, Saint-Martin C, Coste J, Bellanné-Chantelot C, Timsit J (2018) Response to Comment on Dubois-Laforgue et al. Diabetes, associated clinical spectrum, long-term prognosis, and genotype/phenotype correlations in 201 adult patients with hepatocyte nuclear factor 1B (HNF1B) molecular defects. Diabetes Care 2017;40:1436–1443. Diabetes Care 41(1):e8–e9. https://doi.org/10.2337/dci17-0048
Moreno-De-Luca D, SGENE Consortium, Mulle JG, Simons Simplex Collection Genetics Consortium, Kaminsky EB, Sanders SJ, GeneSTAR, Myers SM, Adam MP, Pakula, AT, Eisenhauer NJ, Uhas K, Weik L, Guy L, Care ME, Morel CF, Boni C, Salbert BA, Chandrareddy A, Demmer LA, Chow EW, Surti U, Aradhya S, Pickering DL, Golden DM, Sanger WG, Aston E, Brothman AR, Gliem TJ, Thorland EC, Ackley T, Iyer R, Huang S, Barber JC, Crolla JA, Warren ST, Martin CL, Ledbetter DH (2010) Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am J Hum Genet 87(5):618–630
Clissold RL, Shaw-Smith C, Turnpenny P, Bunce B, Bockenhauer D, Kerecuk L, Waller S, Bowman P, Ford T, Ellard S, Hattersley AT, Bingham C (2016) Chromosome 17q12 microdeletions but not intragenic HNF1B mutations link developmental kidney disease and psychiatric disorder. Kidney Int 90(1):203–211
Dubois-Laforgue D, Bellanné-Chantelot C, Charles P, Jacquette A, Larger E, Ciangura C, Saint-Martin C, Rastel C, Keren B, Timsit J (2017) Monogenic diabetes study group of the Société Francophone du Diabète (SFD). Intellectual disability in patients with MODY due to hepatocyte nuclear factor 1B (HNF1B) molecular defects. Diabetes Metab 43(1):89–92
Raile K, Klopocki E, Holder M, Wessel T, Galler A, Deiss D, Müller D, Riebel T, Horn D, Maringa M, Weber J, Ullmann R, Grüters A (2009) Expanded clinical spectrum in hepatocyte nuclear factor 1b-maturity-onset diabetes of the young. J Clin Endocrinol Metab 94(7):2658–2664
Durand A, Bashamboo A, McElreavey K, Brauner R (2016) Familial early puberty: presentation and inheritance pattern in 139 families. BMC Endocr Disord 16(1):50
Hoekstra AS, Hensen EF, Jordanova ES, Korpershoek E, van der Horst-Schrivers AN, Cornelisse C, Corssmit EP, Hes FJ, Jansen JC, Kunst HP, Timmers HJ, Bateman A, Eccles D, Bovée JV, Devilee P, Bayley JP (2017) Loss of maternal chromosome 11 is a signature event in SDHAF2, SDHD, and VHL-related paragangliomas, but less significant in SDHB-related paragangliomas. Oncotarget 8(9):14525–14536
Bergmann C, von Bothmer J, Ortiz Brüchle N, Venghaus A, Frank V, Fehrenbach H, Hampel T, Pape L, Buske A, Jonsson J, Sarioglu N, Santos A, Ferreira JC, Becker JU, Cremer R, Hoefele J, Benz MR, Weber LT, Buettner R, Zerres K (2011) Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol 22(11):2047–2056
Faguer S, Decramer S, Chassaing N, Bellanné-Chantelot C, Clavas P, Beaufils S, Bessenay L, Lengelé J-P, Dahan K, Ronco P, Devuyst O, Chauveau D (2011) Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int 80(7):768–776
Van der Made CI, Hoorn EJ, de la Faille R, Karaaslan H, Knoers NV, Hoenderop JG, Vargas Poussou R, de Baaij JH (2015) Hypomagnesemia as first clinical manifestation of ADTKD-HNF1B: a case series and literature review. Am J Nephrol 42:85–90
Bingham C, Hattersley AT (2004) Renal cysts and diabetes syndrome resulting from mutations in hepatocyte nuclear factor-1beta. Nephrol Dial Transplant 19(11):2703–2708
Bollée G, Dahan K, Flamant M, Morinière V, Pawtowski A, Heidet L, Lacombe D, Devuyst O, Pirson Y, Antignac C, Knebelmann B (2011) Phenotype and outcome in hereditary tubulointerstitial nephritis secondary to UMOD mutations. Clin J Am Soc Nephrol 6(10):2429–2438
Wolf MT, Beck BB, Zaucke F, Kunze A, Misselwitz J, Ruley J, Ronda T, Fischer A, Eifinger F, Licht C, Otto E, Hoppe B, Hildebrandt F (2007) The uromodulin C744G mutation causes MCKD2 and FJHN in children and adults and may be due to a possible founder effect. Kidney Int 71(6):574–581
Casemayou A, Fournel A, Bagattin A, Schanstra J, Belliere J, Decramer S, Marsal D, Gillet M, Chassaing N, Huart A, Pontoglio M, Knauf C, Bascands JL, Chauveau D, Faguer S (2017) Hepatocyte nuclear factor-1β controls mitochondrial respiration in renal tubular cells. J Am Soc Nephrol 28(11):3205–3217
Edghill EL, Bingham C, Ellard S, Hattersley AT (2006) Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet 43:84–90
Kanda S, Morisada N, Kaneko N, Yabuuchi T, Nawashiro Y, Tada N, Nishiyama K, Miyai T, Sugawara N, Ishizuka K, Chikamoto H, Akioka Y, Iijima K, Hattori M (2016) New-onset diabetes after renal transplantation in a patient with a novel HNF1B mutation. Pediatr Transplant 20(3):467–471
Hecking M, Werzowa J, Haidinger M, Hörl WH, Pascual J, Budde K, Luan FL, Ojo A, de Vries AP, Porrini E, Pacini G, Port FK, Sharif A, Säemann MD, European-New-Onset Diabetes After Transplantation Working Group (2013) Novel views on new-onset diabetes after transplantation: development, prevention and treatment. Nephrol Dial Transplant 28(3):550–566
Furuta H, Furuta M, Sanke T, Ekawa K, Hanabusa T, Nishi M, Sasaki H, Nanjo K (2002) Nonsense and missense mutations in the human hepatocyte nuclear factor-1 beta gene (TCF2) and their relation to type 2 diabetes in Japanese. J Clin Endocrinol Metab 87(8):3859–3863
Johansson BB, Irgens HU, Molnes J, Sztromwasser P, Aukrust I, Juliusson PB, Søvik O, Levy S, Skrivarhaug T, Joner G, Molven A, Johansson S, Njølstad PR (2017) Targeted next-generation sequencing reveals MODY in up to 6.5% of antibody-negative diabetes cases listed in the Norwegian Childhood Diabetes Registry. Diabetologia 60(4):625–635
Bansal V, Gassenhuber J, Phillips T, Oliveira G, Harbaugh R, Villarasa N, Topol EJ, Seufferlein T, Boehm BO (2017) Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals. BMC Med 15(1):213
Iwasaki N, Okabe I, Momoi MY, Ohashi H, Ogata M, Iwamoto Y (2001) Splice site mutation in the hepatocyte nuclear factor-1 beta gene, IVS2nt + 1G > A, associated with maturity-onset diabetes of the young, renal dysplasia and bicornuate uterus. Diabetologia 44(3):387–388
Bingham C, Ellard S, Cole TR, Jones KE, Allen LI, Goodship JA, Goodship TH, Bakalinova-Pugh D, Russell GI, Woolf AS, Nicholls AJ, Hattersley AT (2002) Solitary functioning kidney and diverse genital tract malformations associated with hepatocyte nuclear factor-1beta mutations. Kidney Int 61(4):1243–1251
Oram RA, Edghill EL, Blackman J, Taylor MJ, Kay T, Flanagan SE, Ismail-Pratt I, Creighton SM, Ellard S, Hattersley AT, Bingham C (2010) Mutations in the hepatocyte nuclear factor-1β (HNF1B) gene are common with combined uterine and renal malformations but are not found with isolated uterine malformations. Am J Obstet Gynecol 203(4):364.e1–364.e5. https://doi.org/10.1016/j.ajog.2010.05.022
Acknowledgements
We sincerely thank the participants in this multicenter study and their families.
Funding
This work was kindly supported by the German Society of Pediatric Nephrology and the German Federal Ministry of Research and Education (BMBF, grant 01GM1515).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committees of the University Duisburg-Essen and all collaborating institutions, according to the Declaration of Helsinki. Written informed consent was obtained.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Okorn, C., Goertz, A., Vester, U. et al. HNF1B nephropathy has a slow-progressive phenotype in childhood—with the exception of very early onset cases: results of the German Multicenter HNF1B Childhood Registry. Pediatr Nephrol 34, 1065–1075 (2019). https://doi.org/10.1007/s00467-018-4188-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4188-8