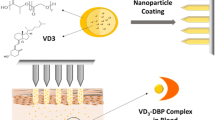
Abstract
Transdermal delivery has been widely studied since it can avoid the effect of first-pass hepatic metabolism and deliver therapeutic agents as systemic or local administration for long period of time. In this study, procaine hydrochloride-loaded poly(lactide-co-glycolide) (PLGA) nanoparticles for transdermal delivery were prepared using a combination of an antisolvent diffusion method with preferential solvation. The physicochemical characteristics and skin permeability were studied. Mean volume diameter of prepared nanoparticles was 99.4 ± 0.8 nm. They were “soft particles” and have negatively charged polyelectrolyte layer on the surface. Ex vivo experiment was carried out using the skin of male Sprague–Dawley rat with Franz diffusion cells. Compared to procaine hydrochloride free molecules, a higher amount of procaine hydrochloride was delivered through rat skin when procaine hydrochloride was loaded in nanoparticles. Moreover, a solution mixture of ethanol and isopropyl myristate, which were used as transdermal enhancers, improved skin permeability of the nanoparticles. In vivo experiment was carried out using male Sprague–Dawley rats aging 8 weeks. Samples were administered on rat abdominal skin (2.5 cm × 2.5 cm). After 9 h, drug concentrations of the skin and muscle under the area of sample administration were measured by using HPLC. Skin accumulation of the drug was increased when drug was included into PLGA nanoparticles, and muscle accumulation was increased by using transdermal enhancer. These results indicate that the nanoparticles that were prepared using a combination of an antisolvent diffusion method with preferential solvation were useful for transdermal delivery.








Similar content being viewed by others
References
Prausniz MR, Langer R (2008) Transdermal drug delivery. Nature Biotech 26:1261–1268. doi:10.1038/nbt.1504
Suhonen TM, Bouwstra JA, Urtti A (1999) Chemical enhancement of percutaneous absorption in relation to stratum corneum structural alterations. J Cont Rel 59:149–161. doi:10.1016/S0168-3659(98)00187-4
Finnin BC, Morgan TM (1999) Transdermal penetration enhancers: applications, limitations, and potential. J Pharm Sci 88:955–958. doi:10.1021/js990154g
Tomoda K, Terashima H, Suzuki K, Inagi T, Terada H, Makino K (2011) Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Coll Surf B 88:706–710. doi:10.1016/j.colsurfb.2011.08.004
Tomoda K, Watanabe A, Suzuki K, Inagi T, Terada H, Makino K (2012) Enhanced transdermal permeability of estradiol using combination of PLGA nanoparticles system and iontophoresis. Coll Surf B 97:84–89. doi:10.1016/j.colsurfb.2012.04.002
Tsujimoto H, Hara K, Huang CC, Yokoyama T, Yamamoto H, Takeuchi H, Kawashima Y, Akagi K, Miwa N (2004) Percutaneous absorption study of biodegradable PLGA nano-spheres via human skin biopsies. J Soc Powder Technol Japan 41:867–875
Miyazaki S, Takahashi A, Kubo W (2003) Poly n-butylcyanoacrylate (PNBCA) nanocapsules as a carrier for NSAIDs: in vitro release and in vivo skin penetration. J Pharm Pharmaceut Sci 6:238–245
Brannon-Peppas L (1995) Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int J Pharm 116:1–9. doi:10.1016/0378-5173(94)00324-X
Takeuchi I, Fukuda K, Kobayashi S, Makino K (2016) Transdermal delivery of estradiol-loaded PLGA nanoparticles using iontophoresis for treatment of osteoporosis. Biomed Mat Eng 27:475–483
Mora-Huertas CE, Fessi H, Elaissari A (2010) Polymer-based nanocapsules for drug delivery. Int J Pharm 385:113–142. doi:10.1016/j.ijpharm.2009.10.018
Boutti S, Bourgeat-Lami E, Zydowicz N (2005) Silica/polyamide nanocomposite synthesis via an original double emulsification process in miniemulsion. Macromol Rapid Commun 26:1860–1865. doi:10.1002/marc.200500518
Tomoda K, Yabuki N, Terada H, Makino K (2014) Surfactant free preparation of PLGA nanoparticles: the combination of antisolvent diffusion with preferential solvation. Coll Surf A 457:88–93. doi:10.1016/j.colsurfa.2014.05.010
Govender T, Stolnik S, Garnett MC, Illum L, Davis SS (1999) PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Cont Rel 57:171–185. doi:10.1016/S0168-3659(98)00116-3
DiPasquale DM, Buono MJ, Kolkhorst FW (2003) Effect of skin temperature on the cholinergic sensitivity of the human eccrine sweat gland. Jpn J Physiol 53:427–430. doi:10.2170/jjphysiol.53.427
Ohshima H (1994) Electrophoretic mobility of soft particles. J Colloid Interface Sci 163:474–483. doi:10.1006/jcis.1994.1126
Makino K, Taki T, Ogura M, Handa S, Nakajima M, Kondo T, Ohshima H (1993) Measurements and analyses of electrophoretic mobilities of RAW117 lymphosarcoma cells and their variant cells. Biophys Chem 47:261–265. doi:10.1016/0301-4622(93)80051-J
Makino K, Ikekita M, Kondo T, Tanuma S, Ohshima H (1994) Change in electrophoretic mobility of HL-60RG cells by apoptosis. Coll Pol Sci 272:487–492. doi:10.1007/BF00659462
Makino K, Fukai F, Hirata S, Ohshima H (1996) Electro-osmotic studies of endothelial cell surface. Coll Surf B 7:235–238. doi:10.1016/0927-7765(96)01303-3
Ohshima H, Kondo T (1989) Approximate analytic expression for the electrophoretic mobility of colloidal particles with surface-charge layers. J Colloid Interface Sci 130:281–282
Ohshima H, Kondo T (1991) On the electrophoretic mobility of biological cells. Biophys Chem 39:191–198. doi:10.1016/0301-4622(91)85021-H
Sahoo SK, Panyam J, Prabha S, Labhasetwar V (2002) Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Cont Rel 82:105–114. doi:10.1016/S0168-3659(02)00127-X
Govender T, Ehtezazi T, Stolnik S, Illum L, Davis SS (1999) Complex formation between the anionic powder (PAA) and a cationic drug (procaine HCl): characterization by microcalorimetric studies. Pharm Res 16:1125–1131. doi:10.1023/A:1018912522342
Mu L, Feng S-S (2003) PLGA/TPGS nanoparticles for controlled release of paclitaxel: effects of the emulsifier and drug loading ratio. Pharm Res 20:1864–1872. doi:10.1023/B:PHAM.0000003387.15428.42
Takeuchi I, Tomoda K, Hamano A, Makino K (2017) Effects of physicochemical properties of poly(lactide-co-glycolide) on drug release behavior of hydrophobic drug-loaded nanoparticles. Colloids Surf A Physicochem Eng Asp 520:771–778. doi:10.1016/j.colsurfa.2017.02.054
Horita D, Todo H, Sugibayashi K (2012) Effect of ethanol pretreatment on skin permeation of drugs. Biol Pharm Bull 35:1343–1348. doi:10.1248/bpb.b12-00293
Engelbrecht TN, Demé B, Dobner B, Neubert RHH (2012) Study of the influence of the penetration enhancer isopropyl myristate on the nanostructure of stratum corneum lipid model membranes using neutron diffraction and deuterium labeling. Skin Pharmacol Physiol 25:200–207. doi:10.1159/000338538
Acknowledgements
This work was supported by the Program for Development of the Strategic Research Center in Private Universities, which is supported by MEXT (2010-2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal care was conducted under the Guidelines for Animal Experimentation of Tokyo University of Science, which are based on the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takeuchi, I., Tomoda, K., Koji, M. et al. Hydrophilic drug-loaded PLGA nanoparticles for transdermal delivery. Colloid Polym Sci 295, 977–983 (2017). https://doi.org/10.1007/s00396-017-4087-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4087-8