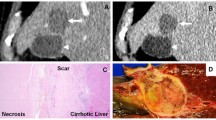
Abstract
Objectives
To evaluate the value of CT attenuation to assess the response to sorafenib in infiltrative/endovascular non-measurable advanced hepatocellular carcinoma (HCC).
Methods
From 2007 to 2014, patients with infiltrative HCC ± tumor-in-vein (TIV) were retrospectively included. Attenuation of tumors and TIV were measured at baseline and follow-up on arterial and portal venous phase CT by two independent radiologists. Attenuation changes (overall and as per Choi criteria) and Child-Pugh score were correlated to overall survival.
Results
Forty patients were included (38 men, 95%). Attenuation of both the tumors and TIV was significantly lower in follow-up CT than on baseline CT (p = 0.002 (arterial), and p = 0.001 (portal) for tumor, and p = 0.004 (arterial) and p < 0.001 (porta) for TIV). Median attenuation of TIV was significantly lower than that of the tumor in follow-up images (p = 0.010). Median OS for the entire cohort was 4 ± 1 months (95% CI: 2.1–5.9), with estimated OS rates at 6, 12, and 24 months of 43%, 29 and 12%, respectively. Baseline and follow-up CT attenuation in tumors and TVI were not correlated with survival. Survival was not significantly increased in patients with Choi criteria >15% CT HU decrease in the tumor and/or TIV during follow-up. Only Child-Pugh A (HR 4.9 (95%CI 2.3–10.7), p < 0.001) was identified as an independent factor of improved survival on multivariate analysis.
Conclusion
Despite significant changes under sorafenib, tumor attenuation of infiltrative/endovascular non-measurable HCC may be of limited value to assess survival in this subgroup of patients with very poor prognosis.
Key Points
• Attenuation of both tumors and tumor-in-vein decreases after sorafenib.
• Attenuation decrease is more marked in the tumor-in-vein than in the tumor.
• Attenuation decrease is not associated with longer overall survival.



Similar content being viewed by others
Abbreviations
- AFP:
-
Alpha-fetoprotein
- BCLC:
-
Barcelona Clinic Liver Cancer
- CT:
-
Computed tomography
- EASL:
-
European Association for the Study of the Liver
- HCC:
-
Hepatocellular carcinoma
- ICC:
-
Intraclass correlation coefficient
- (m)RECIST:
-
(modified) Response Criteria In Solid Tumors
- ROI:
-
Region of interest
- TIV:
-
Tumor-in-vein
References
Forner A, Reig ME, de Lope CR et al (2010) Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 30:61–74
Abou-Alfa GK, Schwartz L, Ricci S et al (2006) Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24:4293–4300
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Reynolds AR, Furlan A, Fetzer DT et al (2015) Infiltrative hepatocellular carcinoma: what radiologists need to know. RadioGraphics 35:371–386
Yopp AC, Mokdad A, Zhu H et al (2015) Infiltrative hepatocellular carcinoma: natural history and comparison with multifocal, nodular hepatocellular carcinoma. Ann Surg Oncol 22(Suppl 3):S1075–S1082
Choi H, Charnsangavej C, Faria SC et al (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25:1753–1759
Choi H, Charnsangavej C, de Castro Faria S et al (2004) CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 183:1619–1628
Ronot M, Bouattour M, Wassermann J et al (2014) Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist 19:394–402
Gavanier M, Ayav A, Sellal C et al (2016) CT imaging findings in patients with advanced hepatocellular carcinoma treated with sorafenib: alternative response criteria (Choi, European Association for the Study of the Liver, and modified Response Evaluation Criteria in Solid Tumor (mRECIST)) versus RECIST 1.1. Eur J Radiol 85:103–112
Salvaggio G, Furlan A, Agnello F et al (2014) Hepatocellular carcinoma enhancement on contrast-enhanced CT and MR imaging: response assessment after treatment with sorafenib: preliminary results. Radiol Med 119:215–221
Kim MJ, Choi JI, Lee JS, Park JW (2011) Computed tomography findings of sorafenib-treated hepatic tumors in patients with advanced hepatocellular carcinoma. J Gastroenterol Hepatol 26:1201–1206
Kneuertz PJ, Demirjian A, Firoozmand A et al (2012) Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol 19:2897–2907
Chapiro J, Duran R, Lin M et al (2015) Early survival prediction after intra-arterial therapies: a 3D quantitative MRI assessment of tumour response after TACE or radioembolization of colorectal cancer metastases to the liver. Eur Radiol 25:1993–2003
Chapiro J, Lin M, Duran R, Schernthaner RE, Geschwind JF (2015) Assessing tumor response after loco-regional liver cancer therapies: the role of 3D MRI. Expert Rev Anticancer Ther 15:199–205
Chapiro J, Wood LD, Lin M et al (2014) Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology 273:746–758
Tacher V, Lin M, Duran R et al (2016) Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach. Radiology 278:275–284
Zhao Y, Duran R, Bai W et al (2018) Which criteria applied in multi-phasic ct can predict early tumor response in patients with hepatocellular carcinoma treated using conventional TACE: RECIST, mRECIST, EASL or qEASL? Cardiovasc Intervent Radiol 41:433–442
Ippolito D, Querques G, Okolicsanyi S, Franzesi CT, Strazzabosco M, Sironi S (2017) Diagnostic value of dynamic contrast-enhanced CT with perfusion imaging in the quantitative assessment of tumor response to sorafenib in patients with advanced hepatocellular carcinoma: a feasibility study. Eur J Radiol 90:34–41
Kaufmann S, Thaiss WM, Schulze M et al (2018) Prognostic value of perfusion CT in hepatocellular carcinoma treatment with sorafenib: comparison with mRECIST in longitudinal follow-up. Acta Radiol 59:765–772
Mule S, Pigneur F, Quelever R et al (2017) Can dual-energy CT replace perfusion CT for the functional evaluation of advanced hepatocellular carcinoma? Eur Radiol. https://doi.org/10.1007/s00330-017-5151-y
Lee S, Kim JH, Lee JH, Lee JH, Han JK (2018) Non-invasive monitoring of the therapeutic response in sorafenib-treated hepatocellular carcinoma based on photoacoustic imaging. Eur Radiol 28:372–381
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Maxime Ronot.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Methodology
• retrospective
• diagnostic or prognostic study
• single-center study
Electronic supplementary material
ESM 1
(DOCX 84 kb)
Rights and permissions
About this article
Cite this article
Koulakian, H., Allaham, W., Vilgrain, V. et al. Non-measurable infiltrative HCC: is post-contrast attenuation on CT a sign of tumor response?. Eur Radiol 29, 4389–4399 (2019). https://doi.org/10.1007/s00330-018-5805-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5805-4