Abstract
Introduction
Metastatic neuroendocrine tumors (NETs) are associated with carcinoid syndrome that is typically characterized by diarrhea, cutaneous flushing and bronchospasm. Treatment with somatostatin analogues (SSA) improves the symptom burden but a significant proportion of patients stop responding to SSA therapy eventually. Novel agents with the potential to effectively control the symptoms are urgently needed.
Methods
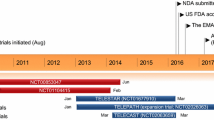
This article reviews an in-depth analysis of the phase I-III clinical trials determining the clinical rationale for the use of tryptophan hydroxylase inhibitor, telotristat ethyl in patients with well-differentiated metastatic NETs and uncontrolled carcinoid syndrome.
Discussion
Telotristat ethyl has already been approved for the treatment of inadequately controlled carcinoid syndrome symptoms in metastatic NET patients on SSA therapy. Results from multiple phase I–III clinical studies of telotristat ethyl therapy have reported a significant decrease in the daily bowel movement frequency, increase in quality of life and the subsequent decrease in annual health costs related to carcinoid syndrome symptoms in NET patients.
Future directions
The associated decrease in urinary 5-hydroxyindoleacetic acid (u5-HIAA) provides evidence that telotristat ethyl effectively decreases serotonin production, and therefore, offers a rationale to investigate this agent to mitigate serotonin-mediated complications in this patient population, especially cardiac valvular disease or mesenteric fibrosis.




Similar content being viewed by others
References
Taal BG, Visser O (2004) Epidemiology of neuroendocrine tumours. Neuroendocrinology 80(Suppl 1):3–7
Legakis I, Saif MW, Syrigos K (2017) Therapeutic challenges in neuroendocrine tumors. Anticancer Agents Med Chem 17(7):902–919. doi:10.2174/1871520617666170213113401
Kulke MH, Mayer RJ (1999) Carcinoid tumors. N Engl J Med 340:858–868
Mocellin S, Nitti D (2013) Gastrointestinal carcinoid: epidemiological and survival evidence from a large population-based study (n = 25 531). Ann Oncol mdt377
Saif MW (2016) Lanreotide for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Opin Pharmacother 17(3):443–56. doi:10.1517/14656566.2016.1127914
Beaumont JL, Cella D, Phan AT, Choi S, Liu Z, Yao JC (2012) Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 41:461–466
Papaxoinis G, Syrigos K, Saif MW (2016) New concepts in the treatment strategy of neuroendocrine tumors: the role of biotherapy. Discov Med 21(117):381–389
Papaxoinis G, Syrigos K, Saif MW (2016) Novel therapeutic approaches and mechanisms in neuroendocrine tumors: the role of targeted agents. Discov Med 21(117):391–402
Khagi S, Saif MW (2014) Gastroenteropancreatic neuroendocrine tumors: hormonal treatment updates. JOP15(2):135–137. doi:10.6092/1590-8577/2287
Oberg K (2000) Interferon in the management of neuroendocrine GEP-tumors: a review. Digestion 62:92
Bainbridge HE, Larbi E, Middleton G (2015) Symptomatic control of neuroendocrine tumours with everolimus. Horm Cancer 6:254–259
Capdevila J, Miranda ID, Obiols G, Tabernero J (2011) Control of carcinoid syndrome with everolimus. Ann Oncol 22:237–239
Lapuerta P, Zambrowicz B, Fleming D, Wheeler D, Sands A (2015) Telotristat etiprate, a novel inhibitor of serotonin synthesis for the treatment of carcinoid syndrome. Clin Investig 5:447 – 56
Kulke MH, O’Dorisio T, Phan A, Bergsland E, Law L, Banks P, Freiman J, Frazier K, Jackson J, Yao JC (2014) Telotristat etiprate, a novel serotonin synthesis inhibitor, in patients with carcinoid syndrome and diarrhea not adequately controlled by octreotide. Endocr Relat Cancer 21:705–714
Melmon KL, Sjoerdsma A, Oates JA, Laster L (1965) Treatment of malabsorption and diarrhea of the carcinoid syndrome with methysergide. Gastroenterology 48:13
Engelman K, Lovenberg W, Sjoerdsma A (1967) Inhibition of serotonin synthesis by para-chlorophenylalanine in patients with the carcinoid syndrome. N Engl J Med 277:1103–1108
Pavel M, Hörsch D, Caplin M, Ramage J, Seufferlein T, Valle J, Banks P, Lapuerta P, Sands A, Zambrowicz B (2015) Telotristat etiprate for carcinoid syndrome: a single-arm, multicenter trial. J Clin Endocrinol Metab 100:1511–1519
Kulke M, Hörsch D, Caplin M, Anthony L, Bergsland E, Oberg K, Welin S, Warner R, Bohas CL, Kunz P (2016) Integrated placebo-controlled safety analysis from clinical studies of telotristat ethyl for the treatment of carcinoid syndrome. Ann Oncol 27:422PD
Khagi S, Saif MW (2015) Pancreatic neuroendocrine tumors: targeting the molecular basis of disease. Curr Opin Oncol 27(1):38–43. doi:10.1097/CCO
Kotteas EA, Syrigos KN, Saif MW (2016) Profile of capecitabine/temozolomide combination in the treatment of well-differentiated neuroendocrine tumors. Onco Targets Ther 9:699–704. doi:10.2147/OTT.S72155
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Saif is on speaker bureau for both Ipsen and Lexicon. Dr. Saif also received Grant funding from Ipsen.
Rights and permissions
About this article
Cite this article
Masab, M., Saif, M.W. Telotristat ethyl: proof of principle and the first oral agent in the management of well-differentiated metastatic neuroendocrine tumor and carcinoid syndrome diarrhea. Cancer Chemother Pharmacol 80, 1055–1062 (2017). https://doi.org/10.1007/s00280-017-3462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3462-y