Abstract
Introduction
Removal of tissue expanders (TE) or implants is a dire consequence of breast reconstruction, and has the potential to halt the reconstructive efforts. Our goals were to characterize a cohort of patients with TE/implant removal, to perform a time-based analysis, and to review the bacteriology associated with explanted devices.
Materials and Methods
Review of a prospectively maintained database was performed to identify patients who underwent TE/implant removal. Patient characteristics, surgical technique, adjuvant therapies, indications, complications, culture results were obtained. Data were analyzed according to timing of explantation.
Results
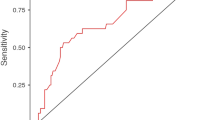
A total of 55 TE and implants were removed in 43 patients. Reasons for explantation were infection (58%), patient request (22%), and wound-related complications (20%). The majority of explantations occurred after 30 days (62%), and after Stage I (81%). Median days to explantation was 62. Patients of older age (p = 0.01) and higher BMI (p = 0.02) were more likely to undergo explantation after Stage I. The most commonly cultured organisms were S. epidermidis (10.9%), S. aureus (10.9%) and P. aeruginosa (10.9%). Antibiotic resistance was commonly encountered for ampicillin, cefazolin, penicillin, and erythromycin.
Conclusion
Infection is the most common reason for explantation after prosthetic breast reconstruction. Patients should be carefully monitored for a prolonged period of time after Stage I, as the majority of explantations occur in this stage but beyond 30 days. For oral treatment, fluoroquinolones and trimethoprim–sulfamethoxazole and for IV treatment a combination of vancomycin or daptomycin with piperacillin–tazobactam or imipenems/carbapenems appear to be appropriate choices according to our culture results.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.


Similar content being viewed by others
References
American Society of Plastic Surgeons Procedural Statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2015/plastic-surgery-statistics-full-report-2015.pdf. Accessed 9 Mar 2017
Disa JJ, Ad-El DD, Cohen SM et al (1999) The premature removal of tissue expanders in breast reconstruction. Plast Reconstr Surg 104(6):1662–1665
Spear SL, Majidian A (1998) Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants: a retrospective review of 171 consecutive breast reconstructions from 1989 to 1996. Plast Reconstr Surg 101(1):53–63
Cohen JB, Carroll C, Tenenbaum MM et al (2015) Breast implant-associated infections: the role of the national surgical quality improvement program and the local microbiome. Plast Reconstr Surg 136(5):921–929
Cordeiro PG, McCarthy CM (2006) A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg 118(4):825–831
Dolen UC, Schmidt AC, Um GT et al (2016) Impact of neoadjuvant and adjuvant chemotherapy on immediate tissue expander breast reconstruction. Ann Surg Onc 23(7):2357–2366
Francis SH, Ruberg RL, Stevenson KB et al (2009) Independent risk factors for infection in tissue expander breast reconstruction. Plast Reconstr Surg 124(6):1790–1796
Khansa I, Hendrick RG, Shore A et al (2014) Breast reconstruction with tissue expanders: implementation of a standardized best-practices protocol to reduce infection rates. Plast Reconstr Surg 134(1):11–18
McCarthy CM, Mehrara BJ, Riedel E et al (2008) Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg 121(6):1886–1892
Viola GM, Baumann DP, Mohan K et al (2016) Improving antimicrobial regimens for the treatment of breast tissue expander-related infections. Plast Reconstr Surg Global Open 4(5):e704–e704
Weichman KE, Levine SM, Wilson SC et al (2013) Antibiotic selection for the treatment of infectious complications of implant-based breast reconstruction. Ann Plast Surg 71(2):140–143
Luce EA, Pierce CE (2015) Lack of validity of the American college of surgeons national surgical quality improvement program database for alloplastic immediate postmastectomy reconstruction. Plast Reconstr Surg 136(3):296e–300e
Fischer JP, Nelson JA, Serletti JM et al (2013) Peri-operative risk factors associated with early tissue expander (TE) loss following immediate breast reconstruction (IBR): a review of 9305 patients from the 2005–2010 ACS-NSQIP datasets. J Plast Reconstr Aesthet Surg 66(11):1504–1512
Fischer JP, Wes AM, Tuggle CT et al (2013) Risk analysis of early implant loss after immediate breast reconstruction: a review of 14,585 patients. J Am Coll Surg 217(6):983–990
Franchelli S, Pesce M, Savaia S et al (2015) Clinical and microbiological characterization of late breast implant infections after reconstructive breast cancer surgery. Surg Infect 16(5):636–644
Alderman A, Gutowski K, Ahuja A et al (2014) Postmastectomy expander implant breast reconstruction guideline work G. ASPS clinical practice guideline summary on breast reconstruction with expanders and implants. Plast Reconstr Surg 134(4):648e–655e
Avraham T, Weichman KE, Wilson S et al (2015) Postoperative expansion is not a primary cause of infection in immediate breast reconstruction with tissue expanders. Breast J 21(5):501–507
Berry T, Brooks S, Sydow N et al (2010) Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol 17(Suppl 3):202–210
Hanwright PJ, Davila AA, Hirsch EM et al (2013) The differential effect of BMI on prosthetic versus autogenous breast reconstruction: a multivariate analysis of 12,986 patients. Breast 22(5):938–945
Hirsch EM, Seth AK, Kim JYS et al (2014) Analysis of risk factors for complications in expander/implant breast reconstruction by stage of reconstruction. Plast Reconstr Surg 134(5):692e–699e
Wang F, Koltz PF, Sbitany H (2014) Lessons learned from the American college of surgeons national surgical quality improvement program database: has centralized data collection improved immediate breast reconstruction outcomes and safety? Plast Reconstr Surg 134(5):859–868
Xue DQ, Qian C, Yang L et al (2012) Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol 38(5):375–381
Woo K-J, Paik JM, Mun G-H et al (2016) Risk factors for complications in immediate expander-implant breast reconstruction for non-obese patients: impact of breast size on complications. Aesthet Plast Surg 40(1):71–78
Achermann Y, Goldstein EJC, Coenye T et al (2014) Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev 27(3):419–440
Khuri SF, Daley J, Henderson W et al (1998) The department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA surgical quality improvement program. Ann Surg 228(4):491–507
Kim JYS, Khavanin N, Jordan SW et al (2014) Individualized risk of surgical-site infection: an application of the breast reconstruction risk assessment score. Plast Reconstr Surg 134(3):351e–362e
Silva AK, Lapin B, Yao KA et al (2015) The effect of contralateral prophylactic mastectomy on perioperative complications in women undergoing immediate breast reconstruction: a NSQIP analysis. Ann Surg Onc 22(11):3474–3480
Ogunleye AA, de Blacam C, Curtis MS et al (2012) An analysis of delayed breast reconstruction outcomes as recorded in the American College of surgeons national surgical quality improvement program. J Plast Reconstr Aesthet Surg 65(3):289–294
Leyngold MM, Stutman RL, Khiabani KT et al (2012) Contributing variables to post mastectomy tissue expander infection. Breast J 18(4):351–356
McCullough MC, Chu CK, Duggal CS et al (2016) Antibiotic prophylaxis and resistance in surgical site infection after immediate tissue expander reconstruction of the breast. Ann Plast Surg 77(5):501–505
Reish RG, Damjanovic B, Austen JWG et al (2013) Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg 131(6):1223–1230
Spear SL, Seruya M (2010) Management of the infected or exposed breast prosthesis: a single surgeon’s 15-year experience with 69 patients. Plast Reconstr Surg 125(4):1074–1084
Spear SL, Howard MA, Boehmler JH et al (2004) The infected or exposed breast implant: management and treatment strategies. Plast Reconstr Surg 113(6):1634–1644
Prince MD, Suber JS, Aya-Ay ML et al (2012) Prosthesis salvage in breast reconstruction patients with periprosthetic infection and exposure. Plast Reconstr Surg 129(1):42–48
Chun JK, Schulman MR (2007) The infected breast prosthesis after mastectomy reconstruction: successful salvage of nine implants in eight consecutive patients. Plast Reconstr Surg 120(3):581–589
Yii N-W, Khoo CTK (2003) Salvage of infected expander prostheses in breast reconstruction. Plast Reconstr Surg 111(3):1087–1092
Meybodi F, Sedaghat N, French J et al (2015) Implant salvage in breast reconstruction with severe peri-prosthetic infection. ANZ J Surg. Nov 17:epub ahead of print
Ariyan S, Martin J, Lal A et al (2015) Antibiotic prophylaxis for preventing surgical-site infection in plastic surgery: an evidence-based consensus conference statement from the American association of plastic surgeons. Plast Reconstr Surg 135(6):1723–1739
Nahabedian MY, Tsangaris T, Momen B et al (2003) Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg 112(2):467–476
Baschnagel AM, Shah C, Wilkinson JB et al (2012) Failure rate and cosmesis of immediate tissue expander/implant breast reconstruction after postmastectomy irradiation. Clin Breast Cancer 12(6):428–432
Cordeiro PG, Pusic AL, Disa JJ et al (2004) Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg 113(3):877–881
Cordeiro PG, Albornoz CR, McCormick B et al (2015) What is the optimum timing of postmastectomy radiotherapy in two-stage prosthetic reconstruction: radiation to the tissue expander or permanent implant? Plast Reconstr Surg 135(6):1509–1517
Tallet AV, Salem N, Moutardier V et al (2003) Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: complications and esthetic results. Int J Radiat Oncol Biol Phys 57(1):136–142
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no disclosures.
Rights and permissions
About this article
Cite this article
Ozturk, C.N., Ozturk, C., Soucise, A. et al. Expander/Implant Removal After Breast Reconstruction: Analysis of Risk Factors and Timeline. Aesth Plast Surg 42, 64–72 (2018). https://doi.org/10.1007/s00266-017-1031-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-017-1031-8