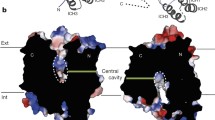
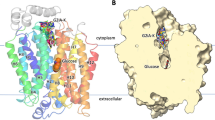
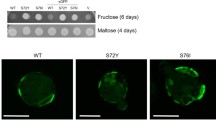
Abstract
Although increased dietary fructose consumption is associated with metabolic impairments, the mechanisms and regulation of intestinal fructose absorption are poorly understood. GLUT5 is considered to be the main intestinal fructose transporter. Other GLUT family members, such as GLUT7 and GLUT9 are also expressed in the intestine and were shown to transport fructose and glucose. A conserved isoleucine-containing motif (NXI) was proposed to be essential for fructose transport capacity of GLUT7 and GLUT9 but also of GLUT2 and GLUT5. In assessing whether human GLUT2, GLUT5, GLUT7, and GLUT9 are indeed fructose transporters, we expressed these proteins in Xenopus laevis oocytes. Stably transfected NIH-3T3 fibroblasts were used as second expression system. In proving the role of the NXI motif, variants p.I322V of GLUT2 and p.I296V of GLUT5 were tested as well. Sugar transport was measured by radiotracer flux assays or by metabolomics analysis of cell extracts by GC–MS. Fructose and glucose uptakes by GLUT7 were not increased in both expression systems. In search for the physiological substrate of GLUT7, cells overexpressing the protein were exposed to various metabolite mixtures, but we failed to identify a substrate. Although urate transport by GLUT9 could be shown, neither fructose nor glucose transport was detectable. Fructose uptake was decreased by the GLUT2 p.I322V variant, but remained unaffected in the p.I296V GLUT5 variant. Thus, our work does not find evidence that GLUT7 or GLUT9 transport fructose or glucose or that the isoleucine residue determines fructose specificity. Rather, the physiological substrate of GLUT7 awaits to be discovered.






Similar content being viewed by others
References
Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ et al (2008) Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem 283(40):26834–26838. doi:10.1074/jbc.C800156200
Augustin R, Carayannopoulos MO, Dowd L, Phay JE, Moley JF, Moley KH (2004) Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem 279(16):16229–16236. doi:10.1074/jbc.M312226200
Barone S, Fussell SL, Singh AK, Lucas F, Xu J, Kim C et al (2009) Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem 284(8):5056–5066. doi:10.1074/jbc.M808128200
Bibert S, Hess SK, Firsov D, Thorens B, Geering K, Horisberger JD, Bonny O (2009) Mouse GLUT9: evidences for a urate uniporter. Am J Physiol Ren Physiol 297(3):F612–F619. doi:10.1152/ajprenal.00139.2009.
Buchs AE, Sasson S, Joost HG, Cerasi E (1998) Characterization of GLUT5 domains responsible for fructose transport. Endocrinology 139(3):827–831. doi:10.1210/endo.139.3.5780
Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO (1992) Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem 267(21):14523–14526
Caulfield MJ, Munroe PB, O’Neill D, Witkowska K, Charchar FJ, Doblado M et al (2008) SLC2A9 is a high-capacity urate transporter in humans. PLoS Med 5(10):e197. doi:10.1371/journal.pmed.0050197.
Cheeseman CI (1993) GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology 105(4):1050–1056
Cheeseman CI (2008) GLUT7: a new intestinal facilitated hexose transporter. Am J Physiol Endocrinol Metab 295(2):E238–E241. doi:10.1152/ajpendo.90394.2008
Corpe CP, Bovelander FJ, Munoz CM, Hoekstra JH, Simpson IA, Kwon O et al (2002) Cloning and functional characterization of the mouse fructose transporter, GLUT5. Biochim Biophys Acta 1576(1–2):191–197
Cuadros-Inostroza A, Caldana C, Redestig H, Kusano M, Lisec J, Peña-Cortés H et al (2009) TargetSearch—a Bioconductor package for the efficient preprocessing of GC-MS metabolite profiling data. BMC Bioinform 10:428. doi:10.1186/1471-2105-10-428
DeBosch BJ, Chi M, Moley KH (2012) Glucose transporter 8 (GLUT8) regulates enterocyte fructose transport and global mammalian fructose utilization. Endocrinology 153(9):4181–4191. doi:10.1210/en.2012-1541
DeBosch BJ, Kluth O, Fujiwara H, Schürmann A, Moley KH (2014) Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun 5:4642. doi:10.1038/ncomms5642
Döring A, Gieger C, Mehta D, Gohlke H, Prokisc, H, Coassin S et al (2008) SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet 40(4):430–436. doi:10.1038/ng.107.
Douard V, Ferraris RP (2008) Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 295(2):E227–E237. doi:10.1152/ajpendo.90245.2008
Inukai K, Katagiri H, Takata K, Asano T, Anai M, Ishihara H et al (1995) Characterization of rat GLUT5 and functional analysis of chimeric proteins of GLUT1 glucose transporter and GLUT5 fructose transporter. Endocrinology 136(11):4850–4857. doi:10.1210/endo.136.11.7588216
Jones HF, Butler RN, Moore DJ, Brooks DA (2013) Developmental changes and fructose absorption in children: effect on malabsorption testing and dietary management. Nutr Rev 71(5):300–309. doi:10.1111/nure.12020
Kane S, Seatter MJ, Gould GW (1997) Functional studies of human GLUT5: effect of pH on substrate selection and an analysis of substrate interactions. Biochem Biophys Res Commun 238(2):503–505. doi:10.1006/bbrc.1997.7204
Kellett GL, Helliwell PA (2000) The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J 350(Pt 1):155–162
Kim HR, Park SW, Cho HJ, Chae KA, Sung JM, Kim JS et al (2007) Comparative gene expression profiles of intestinal transporters in mice, rats and humans. Pharmacol Res Off J Ital Pharmacol Soc 56(3):224–236. doi:10.1016/j.phrs.2007.06.005
Kopka J, Schauer N, Krueger S, Birkemeyer C, Usadel B, Bergmüller E et al (2005) GMD@CSB.DB: the Golm metabolome database. Bioinformatics 21(8):1635–1638. doi:10.1093/bioinformatics/bti236
Li Q, Manolescu A, Ritzel M, Yao S, Slugoski M, Young JD et al (2004) Cloning and functional characterization of the human GLUT7 isoform SLC2A7 from the small intestine. Am J Physiol Gastrointest Liver Physiol 287(1):G236–G242. doi:10.1152/ajpgi.00396.2003
Manolescu A, Salas-Burgos AM, Fischbarg J, Cheeseman CI (2005) Identification of a hydrophobic residue as a key determinant of fructose transport by the facilitative hexose transporter SLC2A7 (GLUT7). J Biol Chem 280(52):42978–42983. doi:10.1074/jbc.M508678200
Manolescu AR, Augustin R, Moley K, Cheeseman C (2007) A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol 24(5–6):455–463. doi:10.1080/09687680701298143
Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K et al (2008) Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet 83(6):744–751. doi:10.1016/j.ajhg.2008.11.001
Miyamoto K, Tatsumi S, Morimoto A, Minami H, Yamamoto H, Sone K et al (1994) Characterization of the rabbit intestinal fructose transporter (GLUT5). Biochem J 303(Pt 3):877–883
Nomura N, Verdon G, Kang HJ, Shimamura T, Nomura Y, Sonoda Y et al (2015) Structure and mechanism of the mammalian fructose transporter GLUT5. Nature 526(7573):397–401. doi:10.1038/nature14909
Patel C, Douard V, Yu S, Gao N, Ferraris RP (2015) Transport, metabolism, and endosomal trafficking-dependent regulation of intestinal fructose absorption. FASEB J Off Publ Feder Am Soc Exp Biol 29(9):4046–4058. doi:10.1096/fj.15-272195.
Röder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H (2014) The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 9(2):e89977. doi:10.1371/journal.pone.0089977
Thompson AMG, Iancu CV, Nguyen, TTH, Kim D, Choe JY (2015) Inhibition of human GLUT1 and GLUT5 by plant carbohydrate products, insights into transport specificity. Sci Rep 5:12804. doi:10.1038/srep12804
Uldry M, Ibberson M, Horisberger JD, Chatton JY, Riederer BM, Thorens B (2001) Identification of a mammalian h(+)-myo-inositol symporter expressed predominantly in the brain. EMBO J 20(16):4467–4477. doi:10.1093/emboj/20.16.4467
Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN et al (2008) SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40(4):437–442. doi:10.1038/ng.106
Acknowledgements
The authors thank Chris Cheeseman for the pGEM-HE GLUT7 and GLUT9 plasmids. Further we thank Daniela Kolmeder for the preparation of GLUT5 cDNA cloned from Caco-2 cells and Martin Klingenspor for supply of pMXs vector. This work was supported by the Deutsche Forschungsgemeinschaft (GRK 1482) to H.W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Karolin Ebert and Maren Ludwig have contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ebert, K., Ludwig, M., Geillinger , K.E. et al. Reassessment of GLUT7 and GLUT9 as Putative Fructose and Glucose Transporters. J Membrane Biol 250, 171–182 (2017). https://doi.org/10.1007/s00232-016-9945-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-016-9945-7