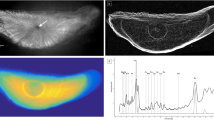
Abstract
The stonelike otoliths from the ears of fish consist of calcium carbonate crystallites embedded in an organic matrix framework. The organic matrix has long been known to play a pivotal role in the biomineralization of otoliths, and different methods have been used to conduct investigations on it. A new sensitive method for the in situ study of the regular variations in the organic matrix composition of serial small yellow croaker otoliths by Raman microspectroscopy and mapping is described. The major collagen bands were always observed around 1,272 cm-1 (amide III) and 1600–1690 cm-1 (amide I), and 1443 and 2800–3100 cm-1 (bending and stretching modes of CH groups, respectively). Aromatic amino acids, such as phenylalanine and tyrosine, were identified at 1,003 cm-1 and at 830 and 853 cm-1. Tryptophan was assigned at 1,555 cm-1, and it was firstly found in otoliths. A regular calcification process in otoliths was observed in Raman spectral mapping results. Corresponding changes were clearly seen in the concentrations of the organic matrix and aragonite (CaCO3) in otoliths.



Similar content being viewed by others
References
Christian S, Manfred B, Elisabeth BN, Jürgen B, Heinz S, Christian R, Teresa N (2003) Science 302:282–286
Tohse H, Mugiya Y (2002) J Fish Biol 61:199–206
Borelli G, Guibbolini ME, Mayer-Gostan N, Priouzeau F, De Pontual H, Allemand D, Puverel S, Tambutte E, Payan P (2003) J Exp Biol 206:2685–2692
Gauldie RW, Sharma SK, Gauldie EVW (1997) Comp Biochem Phys A 118:753–757
Sidgartga P (2005) Nucl Instrum Methods B 229:367–374
Huessy K, Mosegaard H, Jessen F (2004) Can J Fish Aquat Sci 61:1012–1020
Morales-Nin B (1986) S Afr J Mar Sci 4:3–10
Weiner S (1984) Am Zool 24:945–951
Murayama E, Takagi Y, Ohira T, Davis JG, Greene MI, Nagasawa H (2002) Eur J Biochem 269:688–696
Aragae G, Conceicaeo LEC, Dinis HJFMT (2004) Aquaculture 242:589–605
Hedegaard C, Bardeau JF, Chateigner D (2006) J Mollus Stud 72:157–162
Penel G, Delfosse C, Descamps M, Leroy G (2005) Bone 36:893–901
Kazanci M, Roschge P, Paschalis EP, Klaushofer K, Fratzl P (2006) J Struct Biol 156:489–496
Kaczorowska B, Hacura A, Kupka T, Wrzalik R, Talik E, Pastermy G, Matuszewska A (2003) Anal Bioanal Chem 377:1032–1037
Edwards HGM, Villar SEJ, Hassan NFN, Arya N, O’Connor S, Charlton DM (2005) Anal Bioanal Chem 383:713–720
Hope GA, Woods R, Munce CG (2001) Miner Eng 14:1565–1577
Schopf JW, Kudryavtsev AB, Agresti DG, Wdowiak TJ, Czaja AD (2002) Nature 416:73–76
Kim S, Jung S, Zhang CI (1997) Fish Oceanogr 6:1–9
Stevenson DK, Campana SE (1992) Can Spec Publ Fish Aquat Sci 117:41–47
Borelli G, Mayer-Gostan N, Pontual HD, Boeuf G, Payan P (2001) Calcif Tissue Int 69:356–364
Dauphin Y, Dufour E (2003) Comp Biochem Phys A 134:551–561
Xie Y, Zhang DM, Jarori GK, Davisson VJ, Ben-Amotz D (2004) Anal Biochem 332:116–121
Iconomidou VA, Geprgaka ME, Chryssikos GD, Gionis V, Megalofonou P, Hamodrakas SJ (2007) Int J Biol Macromol 41:102–108
Shih S, Weng YM, Chen S, Huang SL, Huang CH, Chen W (2003) Arch Biochem Biophys 420:79–86
Medina-Gutiérrez C, Frausto-Reyes C, Quintanar-Stephano JL, Sato-Berrú R (2004) Spectrochim Acta A 60:2269–2274
Combs A, McCamm K, Autrey D, Laane J, Overman SA, Thomas JGJ (2005) J Mol Struct 735–736:271–278
Kaminaka S, Imamra Y, Shingu M, Kitagawa T, Toyoda T (1999) J Virol Methods 77:117–123
Goodfriend GA, Weidman CR (2001) Geochim Cosmochim Ac 65:1921–1931
Acknowledgements
This work was supported by project grants from the Chinese National Program on Key Basic Research Project (no. 2006CB400601), National Natural Science Foundation of China (no. 40776047), the Science and Technology Committee of Shanghai Municipal (no. 07DJ14003-04) and Excellent Young Teacher in Support Candidates of Shanghai Universities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, F., Cai, W., Sun, Z. et al. Regular variations in organic matrix composition of small yellow croaker (Pseudociaena polyactis) otoliths: an in situ Raman microspectroscopy and mapping study. Anal Bioanal Chem 390, 777–782 (2008). https://doi.org/10.1007/s00216-007-1695-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1695-z