Abstract
The Masimo Radical-7 Pulse CO-Oximeter (Masimo Corp., USA) non-invasively computes hemoglobin concentration (SpHb). SpHb was compared to Co-Oximeter readings (CoOxHb) of arterial samples in surgery patients of the emergency department. Forty-six patients were enrolled. The Masimo R1 25L (revision F and G) adult adhesive sensor was attached to the ring finger of the arterially cannulated hand. Before start, every 30 min during surgery and in the case of severe bleeding SpHb and CoOxHb values were documented. SpHb and post hoc adjusted SpHb (AdSpHb) values were analyzed. Linear regression analysis and Bland–Altman plot for agreement were performed. The detection failure rate of SpHb was 24.5 %. CoOxHb and SpHb showed a strong correlation (r = +0.81), but agreement was moderate [bias (LOA) of −0.6 (−3.0; +1.9)] g/dl. Positive and negative predicted value was 0.49 and 0.69. Exclusion of changes of CoOxHb values ≤1 g/dl resulted in a positive and negative predictive value of 0.66 and 1.00. Post hoc adjustment of the SpHb (AdSpHb) improved linear correlation of CoOxHb and AdSpHb [r = +0.90 (p < 0.001)] but less the agreement [bias (LOA) of CoOxHb and AdSpHb = −0.1 (−2.1/+1.9) g/dl]. SpHb agreed only moderately with CoOxHb values and predicted decreases of CoOxHb only if changes of SpHb ≤ 1.0 g/dl were excluded. The detection failure rate of SpHb was high. At present, additional refinements of the current technology are necessary to further improve performance of non-invasive hemoglobin measurement in the clinical setting.
Similar content being viewed by others
1 Introduction
Trauma-induced hemorrhage and coagulopathy are the major causes of high mortality in severely injured patients [1, 2]. But also during surgical removal of bone metastases or primary large musculoskeletal tumors considerable and life-threatening bleeding can occur.
To estimate the ongoing blood loss in these patients and for implementation of an adequate treatment strategy, periodic hemoglobin (Hb) concentration measurement is one of the useful monitoring tools. Until now point-of-care satellite laboratory blood gas analysis is considered the clinical standard for Hb monitoring as it provides accurate measurements of the Hb concentration [3]. However, this method is invasive, provides only intermittent information about the Hb level during an ongoing process, and is time-consuming.
Continuous real-time and non-invasive monitoring of the Hb concentration promises to be more adapted to the process and would therefore be desirable. Currently the revised Masimo Radical-7™ Pulse CO-Oximeter (Masimo Radical 7 Type RDS-1 Pulse Co-Oximeter and accessories, Masimo Corp., Irvine, CA, USA), a multi-wavelength spectrophotometric technique became commercially available on the medical market for transcutaneous continuous monitoring of the Hb concentration (SpHb). The method of the Radical-7 device is based on the measurement of the differential optical density of seven different wavelengths of light passed through tissue. It provides continuous non-invasive monitoring of several physiologic parameters in addition to the SpHb, including heart rate (RadPR), oxygen saturation (RadSpO2), carboxyhemoglobin saturation (SpCO), and methemoglobin saturation (SpMEt) values. Additionally signal quality is displayed by presenting the low signal identification quality (Low SIQ), Perfusion Index (PI) and Pleth Variability Index (PVI). The functionality and underlying physical laws are described in detail elsewhere [4, 5].
Besides studies in volunteers [6, 7] the capability of SpHb monitoring to measure concentration of Hb in the nail bed capillary in order to estimate systemic Hb concentration has been investigated in patients undergoing caesarean section [8], spine surgery [9], major surgery [10], in the emergency department [11, 12] and in patients having acute upper gastrointestinal bleeding [13]. Yet, the results of the clinical studies are contrary. This might be explained by measurements during periods of hypovolemia. In this condition the central circulatory status is much different from the peripheral circulatory. The physiological effect of volume shifting from the micro- to the macro-circulation could therefore have significant influence on the transcutaneous measured values [14].
The aim of this prospective study is to investigate the reliability of non-invasive continuously measured SpHb by the Masimo Radical-7™ Pulse Co-Oximeter (Masimo Radical 7 Type RDS-1 Pulse CO-Oximeter and accessories; Masimo Corporation, Irvine, CA, USA) under changing circulatory conditions by comparison against simultaneously invasive measured arterial hemoglobin concentrations (CoOxHb) in severe traumatized patients or tumor surgery patients, in which high blood loss was expected.
2 Methods
After approval by the Cantonal Ethics Committee Zurich, Switzerland (Registration Number: KEK-ZH-Nr. 2011-0349) 46 surgical patients were included in this prospective clinical trial. Severity of trauma in the trauma patients was defined according to Butcher and Balogh [15]. With awareness, clear and legally competent patient signed informed consent was obtained before starting the study. In unconscious or legally incapacitated patients at admission to the emergency department signed informed consent occurred after surgery when the patient was aware and legally competent. In continuous unconscious or legally incapacitated patients signed informed consent was given by the relatives. In this case, the relatives had to decide according to the presumed wishes of the patient. Exclusion criteria were age <18 or >80 years, contraindications on ethical grounds, inability to use the upper extremities for monitoring, enrolment into a clinical trial within the last 4 weeks, black skin patients and not German speaking patients or relatives. Withdrawal criteria were refusal to participate in the study during the course of investigation and any technical problems with the device which could be harmful for the patient.
Upon arrival in the theatre of the emergency department patients were prepared with a continuous 2-channel (leads II and V5) electrocardiogram (Delta Infinity Delta XL Kappa Monitor Dräger Medical Systems, Inc, Lübeck, Germany), a continuous invasive arterial blood pressure monitoring via a fluid-filled catheter system (Baxter Healthcare Corp Cardiovascular Group, Irvine, CA, USA) inserted in the radial artery mainly of the non-dominant hand or one of the femoral arties (if upper extremities are not available for catheterization). In hemodynamically unstable patients a triple- or four-lumen central venous line (Arrow International, Reading, PA, USA), was inserted into the right internal jugular or the right subclavian vein and connected with a fluid-filled catheter system (Baxter Healthcare Corp Cardivoascular Group, Irvine, CA, USA). Then a single use finger sensor [MasimoSet rainbow adhesive sensor R1 25 (revision F and G); Masimo, Corporation, Irvine, CA, USA] was attached according to the manufacturer recommendations preferably to the ring finger of the non-dominant hand. Subsequently the finger sensor was connected to the Radical-7 device (Masimo Radical 7 Type RDS-1 Pulse CO-Oximeter and accessories; Masimo Corporation, Irvine, CA, USA). In the case of fingers with nail polish the fingers were cleaned with nail polish remover.
After attaching the finger sensor at the finger the PI value should be >0.75, otherwise, the finger with the highest PI was selected, except the thumb and the pinkie. Induction of general anesthesia was performed by rapid sequence induction using propofol 1.5–2.5 mg/kg, fentanyl 2–20 mcg/kg and Esmeron 0.9 mg/kg per body weight (ED 95). Anesthesia was continued with either propofol 4–12 mg/kg/h or Sevoflurane in a concentration of 0.7–1 minimal alveolar concentration (MAC) combined with bolus injections of fentanyl 2–20 mcg/kg body weight. In hemodynamic unstable patients’ anesthesia was continued using midazolam 0.03–0.1 mg/kg/h and bolus applications of fentanyl 2–20 mcg/kg. Hemodynamic instability was defined as need of norepinephrine in concentrations ≥0.5 µg/kg/min and/or the necessity of both vasopressor and inotropic support under adequate volume resuscitation and a lack of evidence of heart failure.
All data generated by the Radical-7 device including the time points of arterial samples and results of the respective corresponding CO-Oximetry measured arterial CoOxHb values were recorded continuously by an online computing system and additionally in an offline protocol. Measurements were only performed during hemodynamically steady state conditions, defined as mean arterial pressure ≥65 mmHg over a period of 5 min.
Blood samples were drawn before starting surgery, every 30 min and after periods of acute blood loss during surgery. The following data were recorded simultaneously: RadSpO2, SpHb, RadPR, PI, PVI, Low SIQ, arterial CoOxHb, hematocrit (HCT), arterial oxygen saturation (SaO2), arterial partial oxygen pressure (PaO2), arterial carbon dioxide tension (PaCO2), Base excess (BE), HCO3 −, sodium, potassium, chloride, glucose, lactate; central venous oxygenation (ScvO2); pulse oximetry saturation (SpO2), pulse rate (PR), mean arterial pressure (MAP), central venous pressure (CVP), body temperature and respiratory data from the Primus Infinity Anesthesia Ventilator (Dräger Medical Systems, Inc, Lübeck, Germany) end expiratory carbon dioxide tension (ETCO2) and positive end expiratory pressure (PEEP).
CoOxHb, HCT and SaO2 were determined by multi-wavelength pulse oximetry (ABL 825, Radiometer Medical A/S Akandevej 21 DK-2700 Bronshoj, Denmark). The device undergoes quality checks every 8 h, with documented accuracy of ±0 at 8.3 g/dl; ±0.1 at 13.1 and ±0.2 at 19.8 g/dl. Its repeatability error rate is below 2 % over test range of Hb concentrations between 2.5 and 23 g/dl. Before attaching the finger sensor on the finger, 2 and 4 h after removal the finger sensor, at the first and the third day after surgery the peripheral skin perfusion (I = warm and dry, II = cool, and III = cold) and skin status (0 = no reddening, 1 = slight reddening, 2 = reddening, 3 = intensive reddening, and 4 = blister) were assessed and documented.
Based on the recently published Algorithm by Miyashita et al. [16] a post hoc adjustment of the SpHb data was performed. For post hoc adjustment of the SpHb data the difference between the initial CoOxHb and initial SpHb was used and calculated with the formula AdSpHb = RadSpHb − (1st SpHb − 1st CoOxHb).
2.1 Statistics
Sample size calculation was performed with the StatsDirect Statistical Software Version 2.8.0 2013 (StatsDirect Ltd, Altrincham, UK) and revealed a sample size of 95 paired SpHb and CoOxHb measurements to achieve a power of 0.9, α = 0.05 to detect a mean difference (bias) of CoOxHb and SpHb of 0.50 g/dl with an estimated standard deviation (SD) of 1.0 g/dl. The mean difference was determined based on previous investigations. Continuous variables were tested for normal distribution with the Kolmogorov–Smirnow test. Normally distributed data were expressed as mean ± standard deviation (SD); median, interquartile range (IQR; 25th to 75th percentile) and [maximum] were specified when the data were not normally distributed. Categorical variables were presented as number and percentage (%). For subgroup analysis continuous variables were compared using the t test or Mann–Whitney U test, when appropriate. All tests were two-sided and a p-value < 0.05 was determined to be significant.
For all determinants that might have an impact on the fault detection of the SpHb stepwise multiple regressions analysis was performed. Simple regression analysis and Bland–Altman analysis for repeated measurements [17] was performed for the SpHb and CoOxHb, RadSpO2 and SaO2, RadPR and PR data, for post hoc adjusted SpHb (AdSpHb) data, as well for data pairs of CoOxHb, SpHb and AdSpHb values where changes of CoOxHb were > 0.5 g/dl and > 1.0 g/dl. Because adjustment the first measured SpHb to first measured CoOxHb value the data pairs from the first time point were not included in the linear regression and Bland–Altman analysis for repeated measurements for CoOxHb and AdSpHb. Bias (mean absolute difference of the non-dependent and dependent variables) and Limits of agreement (1.96 SD of bias, LOA) were calculated using MedCalc version 13, MedCalc Software bvba. Additionally, Bland–Altman analysis was performed for data pairs of CoOxHb and SpHb, and for data pairs of CoOxHb and AdSpHb at the specific time points. Positive (PPV) and negative (NPV) predictive value were analyzed for SpHb, AdSpHb and SpHb and AdSpHb values where changes of CoOxHb were > 0.5 and > 1.0 g/dl.
3 Results
Forty-six patients were enrolled in the study. A total of 190 blood samples were taken. Eleven patients had to be excluded from analysis. In three of these patients the detection of the SpHb completely failed, in five patients only detection at the first sample time point was possible and in two patients <30 % of all sample time points were detected. One patient had withdrawn his agreement before discharge from the hospital (three data pairs). Stepwise multiple regression analysis revealed significant association of the SpHb by lower CoOxHb (p < 0.001), higher CVP (p = 0.048) and higher volume of crystalloids p = 0.003) on detection failure.
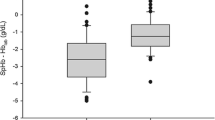
In the remaining 35 included patients 147 blood samples were taken, of which 141 data pairs could be analyzed. Demographic and procedural data are presented in Table 1. Mean ± SD [min; max] of CoOxHb and SpHb were 9.76 ± 2.14 [5.8; 15.6] g/dl and 10.32 ± 1.75 [7.2; 15.2] g/dl, respectively. CoOxHb and SpHb showed a strong linear correlation with r = 0.81 (p < 0.001) (Table 2; Fig. 1a). The bias (LOA) of CoOxHb and SpHb were −0.6 (−3.0/+1.9) g/dl (Table 2; Fig. 1b). Hb (r = 0.58; p < 0.001), HCT (r = 0.58; p < 0.001), CVP (r = 0.47; p < 0.001) MAP (r = 0.20; p = 0.018) and PaCO2 (r = 0.06; p = 0.035) showed an association with the bias of CoOxHb and SpHb. Low CoOxHb values were overestimated and high CoOxHb values were underestimated by the SpHb (r = 0.58, p < 0.001) (Fig. 1b). The PPV and NPV was 0.49 and 0.69 (Table 2). Analysis of data pairs of CoOxHb and SpHb where CoOxHb changes were > 0.5 g/dl (CoOxHb/SpHb 0.5) and > 1 g/dl (CoOxHb/SpHb 1.0) showed no improvement in the agreement (Table 2). The NPV significantly improved to 0.82 and 1.0 whereas the PPV remained only moderate (Table 2).
a Coefficient of determination (r2 = 0.66) with the corresponding linear correlation (r = 0.81) and b Bland–Altman analysis of CoOxHb and SpHb [bias (LOA) = −0.6 (−3.0/+1.9 g/dl)]; n = 141. CoOxHb co-oximetry haemoglobin, SpHb non-invasive measured Haemoglobin by the Radical 7 device, bias mean difference between CoOxHb and SpHb values, LOA Limits of agreement corresponds to 2 SD
Post-hoc adjustment of the SpHb (AdSpHb) significantly improved the linear correlation of CoOxHb and AdSpHb with r = 0.90, p < 0.001 (Table 2; Fig. 2a). Bias (LOA) of CoOxHb and AdSpHb were −0.1 (−2.1/+1.9) g/dl. Low CoOxHb values were underestimated and high CoOXHb values were overestimated by the AdSpHb (r = 0.26, p < 0.001) (Table 2; Fig. 2b). No significant impact factors on the bias of CoOxHb and AdSpHb were found. PPV and NPV were 0.62 and 0.69. Analysing the data pairs of CoOxHb and AdSpHb where CoOxHb changes were > 0.5 g/dl (CoOxHb/AdSpHb 0.5) and > 1 g/dl (CoOxHb/AdSpHb 1.0) no amelioration was found (Table 2). Only the NPV significantly improved to 0.81 and 1.0. Table 3 presents the changes in the agreement of CoOxHb with SpHb and AdSpHb at the different time points of blood sampling during surgery. If only paired values of CoOxHb and AdSpHb to about 120 min of the procedure were analyzed (n = 88), correlation (r = 0.93) was strong and agreement (bias and LOA of −0.1 and −1.8/+1.5 g/dl) was good.
a Coefficient of determination (r2 = 0.82) with the corresponding linear correlation (r = 0.90) and b Bland–Altman analysis of CoOxHb and AdSpHb [bias (LOA) = −0.1(−2.1/+1.9)]; n = 107. CoOxHb co-oximetry haemoglobin, AdSpHb adjusted SpHb based at the AdSpHb = RadSpHb – (1st SpHb – 1st CoOxHb) bias, mean difference between CoOxHb and AdSpHb values, LOA limits of agreement corresponds to 2 SD. Data pairs of the first measurement time point were excluded for Bland–Altman analysis for repeated measurements because of adjustment of these initial measured SpHb values with the initial measured CoOxHb values
Mean ± SD of SaO2 and RadSpO2 were 99.3 ± 1.0 % and 99.3 ± 1.0 %, and for PR and RadPR 73 ± 14 bpm and 74 ± 14 bpm. Agreement of SaO2 and RadSpO2 and PR and RadPR was good with bias and LOA of +0.1 and −1/+2 %; and −0.5 and −4/+3 bpm.
The detection error rate of the Radical-7 device for SpHb was 24.5 % (46 of 187 measuring times) without detection failures for RadSpO2 and RadPR. Neither reddening nor higher degrees of skin irritation were found in any patient during the study period.
4 Discussion
The main findings in this investigation are: (i) CoOxHb and SpHb showed a strong linear correlation, but agreement was only moderate and the prediction for changes of CoOxHb was low. (ii) SpHb reliable predicted decrease of CoOxHb only if changes of SpHb ≤ 1.0 g/dl were excluded. (iii) Posthoc adjustment of the SpHb (AdSpHb) based on the formula published by Miyshita et al. [16] improved correlation but agreement only for a measurement period ≤ 120 min. (iv) In total the detection failure rate of SpHb was 24.5 %.
Investigations comparing continuous noninvasive SpHb measurement with invasive CoOxHb values have been previously performed in volunteers but also in patients undergoing elective and emergency surgery [6–13, 18]. In this study comparison of CoOxHb with the noninvasive measured SpHb showed a strong linear correlation, which was similar or much better compared to the results of other investigations [16, 19–21]. However, the agreement was only moderate if all data pairs of CoOxHb and SpHb were included. The bias and LOA were −0.6 and −3.0 and + 1.9 g/dl. Time point based Bland–Altman analysis presented similar results. Recently, Macknet et al. reported in a comparative study in volunteers undergoing hemodilution a bias ± SD of −0.15 ± 0.92 g/dl and concluded that the SpHb measurement is accurate to 1 g/dl (1SD) compared with laboratory CO-Oximeter [7]. These results seem to be substantially better than those in this study, but changes of hemoglobin were performed under well controlled conditions in young healthy volunteers and interpretation of data, using only the bias, does not show the degree of agreement.
In 127 medical and traumatized patients of an Emergency Department in a Level II trauma center Knutson et al. [12] reported a bias of SpHb and CoOxHb of −0.5 g/dl and LOA of −4.7 and 3.8 g/dl which was beyond the pre-specified clinically acceptable range of ± 1 g/dl. Lamhaut et al. [20] found a strong correlation (r = 0.77) and an excellent accuracy of SpHb and CoOxHb with a bias of −0.02 g/dl but the LOA were very far with −2.75 and 2.70 g/dl in a mixed population of surgical patients. Most recently Tsuei and coworkers reported in 88 adult surgical intensive care unit patients at risk for hemorrhage a bias of SpHb and CoOxHb of +1.5 with LOA of −2.2 to 5.0 g/dl, resulting in a considerable overestimation of CoOxHb [22]. Our results are in accordance with these studies as we also found only a moderate agreement for all data and for the data at the different time points. An important finding in this study was the proportional error in the Bland–Altman analysis, which pointed out that SpHb accuracy is dependent on the amount of change in particular of the CoOxHb and HCT. This dependence was valid for the entire CoOxHb range and resulted in an overestimation of low CoOxHb values and an underestimation of high CoOxHb values by the SpHb. Similar results were found by other investigators [19]. Vos et al. reported this dependence in liver surgery patients, but the relationship between SpHb accuracy and CoOxHb concentration was weaker, and in contrary to our findings only significant for CoOxHb concentrations < 10 g/dl [19]. This finding is of high clinical relevance since decisions about the indication for a blood transfusion require accurate SpHb measurement in lower hemoglobin concentration ranges, especially from the perspective of avoiding unnecessary allogeneic blood transfusions.
The ability of SpHb to reliable detects changes in the CoOxHb concentration during the course in this study was moderate presented by a PPV of 0.49 and NPV of 0.69. In 69 % the changes of CoOxHb and changes of SpHb were in the same direction. Only with exclusion of all data pairs where CoOxHb value changes were ≤ 1 g/dl the NPV was excellent. However the PPV only marginally improved. In accordance to our findings, Applegate et al. reported only moderate ability (r2 = 0.31) of SpHb to reliably detect changes in CoOxHb in patients who underwent abdominal or pelvic surgery [23]. Similar to our results, merely 66 % of the sequential changes in CoOxHb and SpHb were in the same direction. They concluded that there was only a weak correlation between paired changes in CoOxHb and SpHb and that the differences between both measurement modalities were most pronounced in patients with larger blood loss or lower arterial hemoglobin values. However other authors reported acceptable trending of the SpHb. Berkow et al. examined the extent and direction of changes in SpHb when CoOxHb changed by more than 1.5 g/dl between sequential measurements in spine surgery patients. They concluded an acceptable trending of the SpHb, but for verification more studies would be necessary [9]. In major lumbar and low thoracic spine surgery Colquhoun et al. found that 90 % of changes in SpHb were within the limits of acceptance using the polar plot method for assessing trending [24]. For both investigators CoOxHb values changes of 1.0 to 1.5 g/dl were only significant changes. This assumption cannot be applied at the full range of measurable hemoglobin concentrations while acute bleeding. Especially in high risk patients detecting changes of the CoOxHb concentration ≤ 1 g/dl are important in the range of hemoglobin concentrations < 8.0 g/dl for decision making of red cell transfusion. Most recently two investigations performed by Yang et al. [25, 26] in 677 and by Galvagno et al. in 711 trauma patients have shown that SpHb, including both trends and absolute values, did not enhance the ability to predict the need for blood transfusion of the conventional oximetry. The assessment of the reliability of the Radical-7 device as a trend monitor therefore should be near based on the quality criteria and the required accuracy of the commercially available laboratory equipment. However, hemoglobin values will consistently differ within and between various invasive laboratory analyzers. Gehring et al. found a bias of 0.3 g/dl and SD of ±0.2 g/dl between the hemoglobin-cyanide (HiCN) method as gold standard of hemoglobin concentration measurement and a hematology analyzer. They presented a bias of −0.2 g/dl and SD of ±0.3 g/dl between HiCN method and a blood gas analyzer in the same patient [27]. In patients of a surgical intensive care unit Frasca et al. [28] compared 471 blood samples and showed a bias ± SD of 0.9 ± 0.6, 0.0 ± 1.0 and 0.3 ± 1.3 g/dl for a satellite lab CO-Oximeter, a Pulse CO-Oximeter and the HemoCue 301 point of care device. These expectable inaccuracies of the used point-of-care satellite laboratory blood gas analyzer have to be considered in the evaluation of reliability of the non-invasive SpHb measurement method.
After post hoc adjustment the SpHb data of this investigation based on the formula of Miyashita et al. [16] correlation (r = 0.90) and agreement (bias and LOA of −0.1 and −2.1/+1.9 g/dl) improved. Recently Miyashita et al. [16] reported an increase in the correlation from r = 0.89 to r = 0.95 and an improvement the agreement with changing the bias (LOA) from 0.60 to 0.15 (−1.27 to 2.47 and −0.98 to 1.27) for the R1-25 sensor of the radical 7 device. Nearly similar results were found by Isosu et al. [29] in Japanese surgical patients. Adjustment of the SpHb also led to significant improvement of the correlation coefficient from r = 0.76 to r = 0.87 and the agreement of SpHb and Hb changed the bias (LOA) from +0.2 (−2.8 to 3.1) to −0.7 (−2.8 to 1.4). Isosu et al. [29] concluded that in vivo adjustment of the SpHb led to enhanced accuracy in their investigation, although the bias increased and the limits of agreement showed only a slight but not satisfactory improvement. Even though, a moderate improvement of the agreement of CoOxHb and AdSpHb was found in the current investigation, post hoc adjustment of the SpHb did not have any impact on the prediction of AdSpHb to detect changes in CoOxHb compared to the not adjusted SpHb. Time point based Bland–Altman analysis of CoOxHb and AdSpHb clearly shows that the agreement was well to about 120 min after starting the measurements but significantly deteriorated after 120 min of the course. CoOxHb and AdSpHb values within the 120 min period showed a strong correlation with r = 0.93 and good agreement with a bias and LOA of −0.1 and −1.8/+1.5 g/dl, if Bland–Altman analysis for repeated measurements was performed. An important reason for failed improvement of the prediction and trending is the method of post hoc adjustment itself. Initial adjustment at the beginning of the course has important impact on the detection results of all subsequent measurements, because this adjustment corresponds to a calibration and might compensate the physiologically existing difference of erythrocytes amount in the nail bed capillaries and the arteries. This impact is not possible using a post hoc adjustment, in which any influence of an initial calibration on subsequent measures during the process is not possible.
Noteworthy is the high detection failure rate for SpHb of the device. In about 25 % of the measurements the Radical-7 device did not display SpHb value on the screen because of Low SIQ of zero and in almost all cases a PI < 0.75 %. These detection failures were found in particular in multi-traumatized patients, in whom considerable variations in the circulation and severe bleeding were observed. However, not in all cases the Perfusion Index was <1.4. Miyashita et al. [16] reported exclusion of all SpHb values with a PI < 1.4 but did not quantify successful measurement or detection failure rate of the data with PI < 1.4. In this investigation Low SIQ was found in 24.5 % of the measurements and resulted in failed detection of SpHb. However PI > 1.4 was found in 30 % of the Low SIQ cases with failed SpHb detection. Additionally an association of low Hb concentration, high CVP and crystalloid application with the detection failure of SpHb was found. This might be explained that as lower the Hb concentration and as higher the crystalloid influx into the tissue the non-invasive pulsatile detection is likely to be significantly disturbed.
However, especially in bleeding and most hemodynamically unstable patients’ detection failure rate has to be as low as possible, if clinical use of continuous non-invasive hemoglobin measurement for trend monitoring is contemplated.
Limitations of this investigation are the small sample size and comparison the SpHb values with CoOxHb measured by an automated blood gas analyzer as the reference value. Significant intra-device and inter-device variations have been reported by several investigators and should be considered when assessing the results [20, 30]. SpHb values were compared with arterial CoOxHb values. Mokken et al. and Yang et al. [31, 32] reported that hemoglobin concentration measurements derived from arterial blood can be expected to be 0.7–1.0 g/dl lower than those taken from venous blood. This might be explained by the higher plasma concentration in the arterial compared to venous blood. The pulsatile detection of SpHb may collect both the atrial and venous hemoglobin concentration.
More important erythrocytes are not evenly distributed in vessels of the mammal body and the Hb concentrations in the micro- and macro-circulation are different. SpHb measures Hb concentration in the nail bed capillary micro-circulation whereas the Hb concentration in the large vessels macro-circulation is determined by CoOxHb. Especially in capillaries with a diameter <20 µm the relation of red cell to plasma volume is increased compared to those in the macro-circulation. Initial in vivo adjustment the SpHb on the first CoOxHb value might improve the reliability of the SpHb measurement during the process in hemodynamically stable patients. However, during blood loss or plasma loss transcapillary refill follows as a physiological response to the hypovolemia with fluid shift from the micro- to the macro-circulation. In dogs suffering mild, moderate and severe hemorrhagic hypotension Prist et al. have shown that dependent on the degree of shock the volume of transferred fluid represents an ever increasing fraction of total plasma volume and accounts for more than 75 % of plasma volume in pre-terminal stages of shock. Transcapillary refill sustains a relatively fixed level of plasma volume, equivalent to two-third of the initial plasma volume, irrespective of the rate of bleeding [33]. This effect again leads to different time courses of changes in Hb concentration in the nail bad capillaries and the arteries of the macro-circulation and is probably one source of the difference between SpHb and CoOxHb values and the increasing disagreement during the course. If mainly the smallest of capillaries are producing most of the signals SpHb would be rather unaffected by bleeding or dilution due to the Fahraeus–Lindqvist effect. However, in this study patients received timely volume replacement therapy using balanced crystalloids and red cell blood transfusion that affect the physiological response of the micro-circulation.
Last, post hoc adjustment the SpHb off line upon completion of data acquisition is not comparable to an initial in vivo adjustment before starting the course.
In conclusion, this study shows that non-invasive hemoglobin measurement with the Radical-7 device using the MasimoSet rainbow R1 25L (revision F and G) finger sensor showed a high detection failure rate. Detected SpHb values strongly correlated but only moderately agreed with the invasive measured CoOxHb. In severe traumatized and surgical bleeding patients SpHb predicted decreases of CoOxHb only if changes of SpHb ≤ 1.0 g/dl were excluded. At present, additional refinements including the physiology of micro- and macro-circulation covering algorithms are necessary to further improve performance of non-invasive hemoglobin trend measurement in the clinical setting.
References
Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31:1507–11.
Wen Y, Yang H, Wei W, Shan-shou L. The outcomes of 1120 severe multiple trauma patients with hemorrhagic shock in an emergency department: a retrospective study. BMC Emerg Med. 2013;13(Suppl 1):S6.
Stadlbauer V, Wallner S, Stojakovic T, Smolle KH. Comparison of 3 different multianalyte point-of-care devices during clinical routine on a medical intensive care unit. J Crit Care. 2011;26(433):e1–11.
Shamir MY, Avramovich A, Smaka T. The current status of continuous noninvasive measurement of total, carboxy, and methemoglobin concentration. Anesth Analg. 2012;114:972–8.
O’Brien D. Audible alarms in medical equipment. Med Device Diagn Ind. 2006;28:98–103.
Bergek C, Zdolsek JH, Hahn RG. Accuracy of noninvasive haemoglobin measurement by pulse oximetry depends on the type of infusion fluid. Eur J Anaesthesiol. 2013;30:73–9.
Macknet MR, Allard M, Applegate RL 2nd, Rook J. The accuracy of noninvasive and continuous total hemoglobin measurement by pulse CO-Oximetry in human subjects undergoing hemodilution. Anesth Analg. 2010;111:1424–6.
Skelton VA, Wijayasinghe N, Sharafudeen S, Sange A, Parry NS, Junghans C. Evaluation of point-of-care haemoglobin measuring devices: a comparison of Radical-7 pulse co-oximetry, HemoCue((R)) and laboratory haemoglobin measurements in obstetric patients. Anaesthesia. 2013;68:40–5.
Berkow L, Rotolo S, Mirski E. Continuous noninvasive hemoglobin monitoring during complex spine surgery. Anesth Analg. 2011;113:1396–402.
Giraud B, Frasca D, Debaene B, Mimoz O. Comparison of haemoglobin measurement methods in the operating theatre. Br J Anaesth. 2013;111:946–54.
Sjostrand F, Rodhe P, Berglund E, Lundstrom N, Svensen C. The use of a noninvasive hemoglobin monitor for volume kinetic analysis in an emergency room setting. Anesth Analg. 2013;116:337–42.
Knutson T, Della-Giustina D, Tomich E, Wills B, Luerssen E, Reynolds P. Evaluation of a new nonnvasive device in determining hemoglobin levels in emergency department patients. West J Emerg Med. 2013;14:283–6.
Coquin J, Bertarrex A, Dewitte A, Lefevre L, Joannes-Boyau O, Fleureau C, Winnock S, Leuillet S, Janvier G, Ouattara A. Accuracy of determining hemoglobin level using occlusion spectroscopy in patients with severe gastrointestinal bleeding. Anesthesiology. 2013;118:640–8.
Naftalovich R, Naftalovich D. Error in noninvasive spectrophotometric measurement of blood hemoglobin concentration under conditions of blood loss. Med Hypotheses. 2011;77:665–7.
Butcher N, Balogh ZJ. AIS>2 in at least two body regions: a potential new anatomical definition of polytrauma. Injury. 2012;43:196–9.
Miyashita R, Hirata N, Sugino S, Mimura M, Yamakage M. Improved non-invasive total haemoglobin measurements after in vivo adjustment. Anaesthesia. 2014;69:752–6.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Hahn RG, Li Y, Zdolsek J. Non-invasive monitoring of blood haemoglobin for analysis of fluid volume kinetics. Acta Anaesthesiol Scand. 2010;54:1233–40.
Vos JJ, Kalmar AF, Struys MM, Porte RJ, Wietasch JK, Scheeren TW, Hendriks HG. Accuracy of non-invasive measurement of haemoglobin concentration by pulse co-oximetry during steady-state and dynamic conditions in liver surgery. Br J Anaesth. 2012;109:522–8.
Lamhaut L, Apriotesei R, Combes X, Lejay M, Carli P, Vivien B. Comparison of the accuracy of noninvasive hemoglobin monitoring by spectrophotometry (SpHb) and HemoCue(R) with automated laboratory hemoglobin measurement. Anesthesiology. 2011;115:548–54.
Causey MW, Miller S, Foster A, Beekley A, Zenger D, Martin M. Validation of noninvasive hemoglobin measurements using the Masimo Radical-7 SpHb Station. Am J Surg. 2011;201:592–8.
Tsuei BJ, Hanseman DJ, Blakeman MJ, Blakeman TC, Yang SH, Branson RD, Gerlach TW. Accuracy of noninvasive hemoglobin monitoring in patients at risk for hemorrhage. J Trauma Acute Care Surg. 2014;77:S134–9.
Applegate RL 2nd, Barr SJ, Collier CE, Rook JL, Mangus DB, Allard MW. Evaluation of pulse cooximetry in patients undergoing abdominal or pelvic surgery. Anesthesiology. 2012;116:65–72.
Colquhoun DA, Forkin KT, Durieux ME, Thiele RH. Ability of the Masimo pulse CO-Oximeter to detect changes in hemoglobin. J Clin Monit Comput. 2012;26:69–73.
Yang SH, Peter F, Anazodo A, Gao C, Chen H, Wade Ch., Hartsky L, Miller C, Imle C, Fang R, Mackenzie CF. Trends of hemoglobin oximetry: do they help predict blood transfusion during trauma patient resuscitation? Anesth Analg. 2015.
Galvagno SM Jr, Hu P, Yang S, Gao C, Hanna D, Shackelford S, Mackenzie C. Accuracy of continuous noninvasive hemoglobin monitoring for the prediction of blood transfusions in trauma patients. J Clin Monit Comput. 2015;29:815–21.
Gehring H, Hornberger C, Dibbelt L, Rothsigkeit A, Gerlach K, Schumacher J, Schmucker P. Accuracy of point-of-care-testing (POCT) for determining hemoglobin concentrations. Acta Anaesthesiol Scand. 2002;46:980–6.
Frasca D, Dahyot-Fizelier C, Catherine K, Levrat Q, Debaene B, Mimoz O. Accuracy of a continuous noninvasive hemoglobin monitor in intensive care unit patients. Crit Care Med. 2011;39:2277–82.
Isosu T, Obara S, Hosono A, Ohashi S, Nakano Y, Imaizumi T, Mogami M, Murakawa M. Validation of continuous and noninvasive hemoglobin monitoring by pulse CO-oximetry in Japanese surgical patients. J Clin Monit Comput. 2013;27:55–60.
Gehring H, Duembgen L, Peterlein M, Hagelberg S, Dibbelt L. Hemoximetry as the “gold standard”? Error assessment based on differences among identical blood gas analyzer devices of five manufacturers. Anesth Analg. 2007;105:S24–30.
Mokken FC, van der Waart FJ, Henny CP, Goedhart PT, Gelb AW. Differences in peripheral arterial and venous hemorheologic parameters. Ann Hematol. 1996;73:135–7.
Yang ZW, Yang SH, Chen L, Qu J, Zhu J, Tang Z. Comparison of blood counts in venous, fingertip and arterial blood and their measurement variation. Clin Lab Haematol. 2001;23:155–9.
Prist R, Rocha-e-Silva M, Scalabrini A, Coelho IJ, Franca ES, Meneghetti C, Braga GA. A quantitative analysis of transcapillary refill in severe hemorrhagic hypotension in dogs. Shock. 1994;1:188–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Werner Baulig has received honoraria or travel support for consulting or lecturing from the following companies: CSL Behring Schweiz, Zurich, Switzerland, Fresenius Kabi (Schweiz) AG, Oberndorf, Switzerland; Orion Pharma AG, Zug, Switzerland; B. Braun Medical AG, Sempach, Switzerland. Masimo supported the trial by providing the consumables and the Radical 7 device free of charge. The company had no role in the design, conduction, collection and analysis of the data, or in the drafting of the manuscript. Burkhardt Seifert has no conflict of interest. Donat R. Spahn The academic department is receiving grant support from the Swiss National Science Foundation, Berne, Switzerland (grant numbers: 33CM30_124117 and 406440-131268), the Swiss Society of Anesthesiology and Reanimation (SGAR), Berne, Switzerland (no grant numbers are attributed), the Swiss Foundation for Anesthesia Research, Zurich, Switzerland (no grant numbers are attributed), Bundesprogramm Chancengleichheit, Berne, Switzerland (no grant numbers are attributed), CSL Behring, Berne, Switzerland (no grant numbers are attributed), Vifor SA, Villars-sur-Glâne, Switzerland (no grant numbers are attributed). Dr. Spahn was the chairman of the ABC Faculty and a member of the ABC Trauma Faculty which both are managed by Thomson Physicians World GmbH, Mannheim, Germany and sponsored by an unrestricted educational grant from Novo Nordisk A/S, Bagsvärd, Denmark. In the past 5 years, Dr. Spahn has received honoraria or travel support for consulting or lecturing from the following companies: Abbott AG, Baar, Switzerland, AstraZeneca AG, Zug, Switzerland, Bayer (Schweiz) AG, Zürich, Switzerland, Baxter S.p.A., Roma, Italy, B. Braun Melsungen AG, Melsungen, Germany, Boehringer Ingelheim (Schweiz) GmbH, Basel, Switzerland, Bristol-Myers-Squibb, Rueil-Malmaison Cedex, France, CSL Behring GmbH, Hattersheim am Main, Germany and Bern, Switzerland, Curacyte AG, Munich, Germany, Ethicon Biosurgery, Sommerville, New Jersey, USA, Fresenius SE, Bad Homburg v.d.H., Germany, Galenica AG, Bern, Switzerland (including Vifor SA, Villars-sur-Glâne, Switzerland), GlaxoSmithKline GmbH & Co. KG, Hamburg, Germany, Janssen-Cilag AG, Baar, Switzerland, Novo Nordisk A/S, Bagsvärd, Denmark, Octapharma AG, Lachen, Switzerland, Organon AG, Pfäffikon/SZ, Switzerland, Oxygen Biotherapeutics, Costa Mesa, CA, Pentapharm GmbH (now tem Innovations GmbH), Munich, Germany, Roche Pharma (Schweiz) AG, Reinach, Switzerland and Schering-Plough International, Inc., Kenilworth, New Jersey, USA. Oliver M. Theusinger has received honoraria or travel support for consulting or lecturing from the following companies: CSL Behring Schweiz, Zurich, Switzerland, Vifor SA, Villars-sur-Glâne, Switzerland, Roche Pharma (Schweiz) AG, Reinach, Switzerland, Pentapharm AG, München, Germany, TEM International GmbH, München, Germany.
Financial disclosure
Masimo Corporation 52 Discovery Irvine, CA, US supported the trial by providing the consumables and the Radical 7 device free of charge.
Rights and permissions
About this article
Cite this article
Baulig, W., Seifert, B., Spahn, D.R. et al. Accuracy of non-invasive continuous total hemoglobin measurement by Pulse CO-Oximetry in severe traumatized and surgical bleeding patients. J Clin Monit Comput 31, 177–185 (2017). https://doi.org/10.1007/s10877-015-9816-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9816-2