Abstract
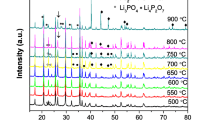
Flake-like LiFePO4 were hydrothermally synthesized in an organic-free solution at heating rates of 0.5, 1.5, 3, and 5 °C min−1. The heating rate has a marked influence on crystal morphology but scarcely on phase purity. The reason for morphology variations is discussed based upon the solubility of precursors Li3PO4 and Fe3(PO4)2·8H2O. The optimum heating rate for hydrothermal synthesis of LiFePO4 is 3 °C min−1. The as-synthesized material exhibits a high specific capacity, excellent rate capability, good low-temperature performance and Li+ diffusivity after carbon coating, all of which could be ascribed to shortened Li+ diffusion distance and higher crystallization degree of the crystalline.






Similar content being viewed by others
References
Ferrari S, Lavall RL, Capsoni D, Quartarone E, Magistris A, Mustarelli P, Canton P (2010) Influence of particle size and crystal orientation on the electrochemical behavior of carbon-coated LiFePO4. J Phys Chem C 114:12598–12603
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194
Yu F, Zhang L, Li Y, An Y, Zhu M, Dai B (2014) Mechanism studies of LiFePO4 cathode material: lithiation/delithiation process, electrochemical modification and synthetic reaction. RSC Adv 4:54576–54602
Lu Z, Chen H, Robert R, Zhu BYX, Deng J, Wu L, Chung CY, Grey CP (2011) Citric acid- and ammonium-mediated morphological transformations of olivine LiFePO4 particles. Chem Mater 23:2848–2859
Zhang YD, Li Y, Xia XH, Wang XL, Gu CD, Tu JP (2015) High-energy cathode materials for Li-ion batteries: a review of recent developments. Sci China Technol Sci 58:1809–1828
Myung S-T, Amine K, Sun Y-K (2015) Nanostructured cathode materials for rechargeable lithium batteries. J Power Sources 283:219–236
Dou S (2015) Review and prospects of Mn-based spinel compounds as cathode materials for lithium-ion batteries. Ionics 21:3001–3030
Chen G, Song X, Richardson TJ (2006) Electron microscopy study of the LiFePO4 to FePO4 phase transition. J Electrochem Soc 9:A295–A298
Wang J, Sun X (2011) Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ Sci 5:5163–5185
Morgan D, Van der Ven A, Ceder G (2004) Li conductivity in Li x MPO4 (M = Mn, Fe, Co, Ni) olivine materials. Electrochem Solid St 7:A30–A32
Prosini PP, Lisi M, Zane D, Pasquali M (2002) Determination of the chemical diffusion coefficient of lithium in LiFePO4. Solid State Ionics 148:45–51
Gaberscek M, Dominko R, Jamnik J (2007) Is small particle size more important than carbon coating? An example study on LiFePO4 cathodes. Electrochem Commun 9:2778–2783
Wang K-X, Li X-H, Chen J-S (2014) Surface and interface engineering of electrode materials for lithium-ion batteries. Adv Mater 27:527–545
Wang Y, Wang Y, Hosono E, Wang K, Zhou H (2008) The design of a LiFePO4/carbon nanocomposite with a core-shell structure and its synthesis by an in situ polymerization restriction method. Angew Chem 120:7571–7575
Paolella A, Turner S, Bertoni G, Hovington P, Flacau R, Boyer C, Feng ZM, Colombo M, Marras S, Prato M, Manna L, Guerfi A, Demopoulos GP, Armand M, Zaghib K (2016) Accelerated removal of Fe-antisite defects while nanosizing hydrothermal LiFePO4 with Ca2+. Nano Lett 16:2692–2697
Li H, Zhou H (2012) Enhancing the performances of Li-ion batteries by carbon-coating: present and future. Chem Commun 48:1201–1217
Bewlay SL, Konstantinov K, Wang GX, Dou SX, Liu HK (2004) Conductivity improvements to spray-produced LiFePO4 by addition of a carbon source. Mater Lett 58:1788–1791
Chen M, Ma Q, Wang C, Sun X, Wang L, Zhang C (2014) Amphiphilic carbonaceous material-intervened solvothermal synthesis of LiFePO4. J Power Sources 263:268–275
Chen Z, Du B, Xu M, Zhu H, Li L, Wang W (2013) Polyacene coated carbon/LiFePO4 cathode for Li ion batteries: understanding the stabilized double coating structure and enhanced lithium ion diffusion kinetics. Electrochim Acta 109:262–268
Johnson ID, Blagovidova E, Dingwall PA, Brett DJL, Shearing PR, Darr JA (2016) High power Nb-doped LiFePO4 Li-ion battery cathodes; pilot-scale synthesis and electrochemical properties. J Power Sources 326:476–481
Chekannikov AA, Kapaev RR, Novikova SA, Kulova TL, Skundin AM, Yaroslavtsev AB (2016) Research of lithium iron phosphate as material of positive electrode of lithium-ion battery. Int J Electrochem Sci 11:2219–2229
He R, Liu Z, Zhang L, Guo R, Yang S (2016) Electrochemical properties of V and Ti co-doping Li1.02FePO4/C material prepared by solid-state synthesis route. J Alloy Compd 662:461–466
Chen J, Wang S, Whittingham MS (2007) Hydrothermal synthesis of cathode materials. J Power Sources 174:442–448
Chen Z, Zhao Q, Xu M, Li L, Duan J, Zhu H (2015) Electrochemical properties of self-assembled porous micro-spherical LiFePO4/PAS composite prepared by spray-drying method. Electrochim Acta 186:117–124
Islam M, Ur SC, Yoon MS (2015) Improved performance of porous LiFePO4/C as lithium battery cathode processed by high energy milling comparison with conventional ball milling. Curr Appl Phys 15:541–546
Zhao N, Li Y, Zhao X, Zhi X, Liang G (2016) Effect of particle size and purity on the low temperature electrochemical performance of LiFePO4/C cathode material. J Alloy Compd 683:123–132
Song Q, Ou X, Wang L, Liang G, Wang Z (2011) Effect of pH value on particle morphology and electrochemical properties of LiFePO4 by hydrothermal method. Mater Res Bull 46:1398–1402
Ou X, Pan L, Gu H, Wu Y, Lu J (2012) Temperature-dependent crystallinity and morphology of LiFePO4 prepared by hydrothermal synthesis. J Mater Chem 22:9064–9068
Meligrana G, Gerbaldi C, Tuel A, Bodoardo S, Penazzi N (2006) Hydrothermal synthesis of high surface LiFePO4 powders as cathode for Li-ion cells. J Power Sources 160:516–522
Liu Y, Gu J, Zhang J, Yu F, Wang J, Nie N, Li W (2015) LiFePO4 nanoparticles growth with preferential (010) face modulated by Tween-80. RSC Adv 5:9745–9751
Tian R, Liu G, Liu H, Zhang L, Gu X, Guo Y, Wang H, Sun L, Chu W (2015) Very high power and superior rate capability LiFePO4 nanorods hydrothermally synthesized using tetraglycol as surfactant. RSC Adv 5:1859–1866
Di Lupo F, Meligrana G, Gerbaldi C, Bodoardo S, Penazzi N (2015) Surfactant-assisted mild solvothermal synthesis of nanostructured LiFePO4/C cathodes evidencing ultrafast rate capability. Electrochim Acta 156:188–198
Chen Z, Xu M, Du B, Zhu H, Xie T, Wang W (2014) Morphology control of lithium iron phosphate nanoparticles by soluble starch-assisted hydrothermal synthesis. J Power Sources 272:837–844
Liu Y, Gu J, Zhang J, Wang J, Nie N, Fu Y, Li W, Yu F (2015) Controllable synthesis of nano-sized LiFePO4/C via a high shear mixer facilitated hydrothermal method for high rate Li-ion batteries. Electrochim Acta 173:448–457
Ou X, Gu H, Wu Y, Lu J, Zheng Y (2013) Chemical and morphological transformation through hydrothermal process for LiFePO4 preparation in organic-free system. Electrochim Acta 96:230–236
Fisher CAJ, Islam MS (2008) Surface structures and crystal morphologies of LiFePO4: relevance to electrochemical behaviour. J Mater Chem 18:1209–1215
Amal A-B, Tomson MB (1994) The temperature dependence of the solubility product constant of vivianite. Geochim Cosmochim Acta 58:5373–5378
Park KY, Park I, Kim H, Yoon G, Gwon H, Cho Y, Yun YS, Kim JJ, Lee S, Ahn D, Kim Y, Hwang I, Yoon WS, Kang K (2016) Lithium-excess olivine electrode for lithium rechargeable batteries. Energy Environ Sci 9:2902–2915
Nishimura S, Kobayashi G, Ohoyama K, Kanno R, Yashima M, Yamada A (2008) Experimental visualization of lithium diffusion in Li x FePO4. Nat Mater 7:707–711
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 11252 kb)
Rights and permissions
About this article
Cite this article
Min, C., Ou, X., Shi, Z. et al. Effects of heating rate on morphology and performance of lithium iron phosphate synthesized by hydrothermal route in organic-free solution. Ionics 24, 1285–1292 (2018). https://doi.org/10.1007/s11581-017-2287-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2287-3