Abstract
Endophytic bacteria associated with sweet potato plants (Ipomoea batatas (L.) Lam.) were isolated, identified and tested for their ability to fix nitrogen, produce indole acetic acid (IAA), and exhibit stress tolerance. Eleven different strains belonging to the genera, Enterobacter, Rahnella, Rhodanobacter, Pseudomonas, Stenotrophomonas, Xanthomonas and Phyllobacterium, were identified. Four strains were shown to produce IAA (a plant growth hormone) and one strain showed the ability to grow in nitrogen free medium and had the nitrogenase subunit gene, nifH. To determine if IAA production by the endophytes had any role in protecting the cells against adverse conditions, different stress tests were conducted. The IAA producer grew well in the presence of some antibiotics, UV and cold treatments but the response to pH, osmotic shock, thermal and oxidative treatments was the same for both the IAA producer and the no IAA producer. To determine if IAA produced by the strains was biologically relevant to plants, cuttings of poplar were inoculated with the highest IAA producing strain. The inoculated cuttings produced roots sooner and grew more rapidly than uninoculated cuttings. These studies indicate that endophytes of sweet potato plants are beneficial to plant growth.
Similar content being viewed by others
Introduction
Endophytes are microorganisms that reside within the inner parts of plants without causing any disease symptoms (Hallmann et al. 1997). Cultivable endophytic communities can be isolated after surface sterilization of the plant material. Endophytes exist in a range of tissue types within a broad range of plants, colonizing the plant systemically with bacterial colonies and biofilms, residing latently in intercellular spaces, inside the vascular tissue or within cells (Ulrich et al. 2008). Although the plant-endophyte interaction has not been fully understood, it has been reported that many isolates provide beneficial effects to their hosts like preventing disease development by synthesizing novel compounds and antifungal metabolites. Investigations of biodiversity of endophyte strains for novel metabolites may identify new drugs for the treatment of human, plant and animal diseases (Strobel et al. 2004). Several bacterial endophytes have been shown to support plant growth and increase nutrient uptake by providing phytohormones (Jacobson et al. 1994), low molecular weight compounds (Frommel et al. 1991), enzymes (Glick et al. 1998), antimicrobial substances like antibiotics (Bangera and Thomashow 1996) and siderophores (O’Sullivan and O’Gara 1992). Some endophytes offer increased resistance to pathogens thus making them ideal candidates for biological control (Madhaiyan et al. 2004). Other beneficial effects of endophytes to plants include nitrogen fixation (Barraquio et al. 1997), increased drought resistance (Nowak et al. 1995), thermal protection (Redman et al. 2002), survival under osmotic stress (Creus et al. 1998) and more recently, their potential for enhanced degradation of several pollutants has also been investigated (Reviewed in Doty 2008).
Sweet potato (Ipomoea batatas (L.) Lam.) is a resilient, easily propagated crop, growing well in infertile and nitrogen (N) poor soils. Over 95% of the global sweet potato crop is produced in developing countries (Reiter et al. 2003). It has also shown potential to tolerate and absorb heavy metal pollutants like lead, iron and cadmium, as well as mixed pollutants contained in landfill leachate (deAraujo et al. 2004). In this paper we focus on the culturable bacterial endophytes of sweet potato and their possible contribution in the growth of the plant.
Materials and methods
Plant material and isolation of bacterial endophytes
Sweet potatoes were purchased from a grocery store in Seattle, WA. In order to generate plants, in each sweet potato a few inoculating sticks were inserted and the potato was hung in a beaker of water, and placed under light. When shoots emerged from the potato and reached approximately 15 cm in height, the rooted 10d old plants were transferred to pots containing non sterile, 1:1 (w/w) ProMix (Premier Horticulture, Quakertown, PA) and perlite (Therm-O-Rock, Chandler, AZ). Four sweet potatoes were used to generate the plants and were designated as SP1, SP2, SP3 and SP4. Cuttings of ∼8 cm in length were surface sterilized with 10% bleach for 10 min, 1% iodophor for 5 min and rinsed several times with sterile water. The ends of the explants were removed and the stems were incubated in the light for 1 w on Murashige and Skoog (MS) medium containing 0.1% phytagar (Murashige and Skoog 1962). Resulting bacteria were subcultured overnight on Luria-Bertani medium with mannitol glutamate and added salts (MG/L) (Chilton et al. 1974). Single colonies were selected (Table 1), grown in MG/L medium overnight, and stored in 33% glycerol at −80°C for subsequent characterization. To identify the role of sweet potato endophytes in plant growth, we generated some internally sterile plants by propagating them in MS medium containing antibiotics. The concentration of the antibiotics used was determined from previous experiments (data not shown) that was enough to kill the endophytes and not harm the plant. Validation of sterility was done by crushing the plants in sterile conditions and growing the extract on MS plates and checking for bacterial growth.
Bacteria identification using 16S rRNA sequences
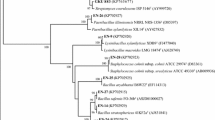
Genomic DNA was prepared using standard methods (Ausubel et al. 1995) with a cell lysis performed at 68°C for 30 min (Doty et al. 2005). PCR was performed using the universal 16S rRNA primers - 8F (5′- AGAGTTTGATCCTGGCTCAG-3′) and 1492 (5′- GGTTACCTTGTTACGACTT-3′) as described previously (Doty et al. 2005). The thermocycler program was 94°C for 15 s, 44°C for 15 s, 72°C for 30 s and it was repeated for 24 cycles. Samples were subjected to electrophoresis in a 1% agarose gel. The 1.5 Kb PCR products were purified using Qiagen gel extraction kit (Qiagen; Valencia, CA) subcloned into pGEM T Easy (Promega; Madison, WI) and sequenced using T7 and SP6 primer sets on the vector by the University of Washington Biochemistry Department Sequencing Facility using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems; Carlsbad, CA) and an ABI3730 XL sequencer (Applied Biosystems). The obtained sequences were assembled using the Seqman software (DNA STAR Inc.) and sequence comparisons with public databases were performed via the Internet at the National Center for Biotechnology Information site (http://www.ncbi.nlm.nih.gov/), by employing the BLASTN algorithm (Altschul et al. 1997). Sequences were submitted to GenBank and accession numbers were obtained (Fig. 1). Phylogenetic analysis was done using CLUSTAL W software (Thompson et al. 1994). Evolutionary distance matrix were constructed using the algorithm of Jukes and Cantor (Thompson et al. 1994) and the evolutionary trees for the data sets were inferred from the neighbor-joining method (Saitou and Nei 1987) by using MEGA version 4.0.1 (Tamura et al. 2007).
Phylogenetic relationships among 11 isolates of endophytic bacteria (marked in bold) obtained in culture from sweet potato plants and the related bacterial species. The Neighbor-Joining dendogram was derived from a 16S rRNA sequence distance matrix (Jukes-Cantor). Bootstrap confidence levels greater than 95% are indicated at the internodes. GenBank accession numbers are shown in parentheses. Bar, 2 substitutions per 100 nucleotide positions
Growth on nitrogen- limited medium
Isolates from the frozen stocks were streaked on MG/L medium and incubated at 30°C overnight. Isolated colonies were streaked on nitrogen free MS medium (Caisson MSP007; http://www.caissonlabs.com) containing either 3% sucrose or glucose as the carbon source, and growth was assayed after 2 d. For growth curve assays, a homogeneous inoculum from the isolated colonies in the nitrogen free MS medium was used to inoculate 25 ml of nitrogen free MS medium with either 3% sucrose or glucose. Growth was monitored using spectrophotometer and measuring the optical density at 600 nm. Statistical analysis was done using split plot ANOVA (Intercooled Stata 10.0, StataCorp LP, College Station, TX) in order to account for the multiple measures taken over time on each flask, and the replicated flasks for each sample.
Cloning of nitrogenase gene fragments
Genomic DNA from isolates SPa and SPb was subjected to nested PCR. This technique allows an additional level of specificity utilizing two different sets of primers, one set internal to the other. PCR primers to amplify the highly conserved region of the nitrogenase subunit H gene (nifH) were used as described (Burgmann et al. 2004). The first set of degenerate primers (nifH-universal for A site and reverse site)—forward primer 5′-GCIWTITAYGGNAARGGNGG- 3′ and reverse primer 5′- GCRTAIABNGCCATCATYTC- 3′- amplified a 464 bp region of the nitrogenase gene (Burgmann et al. 2004). Several Epicenter FailSafe PCR buffer series (A, B, E, F, and G) were tried. Epicenter Pre-Mix Buffer A was then selected. The thermocycler program was 5 min at 95°C and then 11 s at 94°C, 8 s at 48°C, and 10 s at 72°C for 40 cycles, followed by a polishing step for 10 min at 72°C. One microlitre of the 25 μl sample was then used in the nested reactions. The series of nested primers described in Burgmann et al. (2004) amplified a 371 bp sub region of the nifH gene. The 371 bp products were gel purified and subcloned into pGEM T Easy. Azotobacter vinelandii DNA served as a positive control and for negative control water was used instead of DNA. Sequencing of the inserts in both directions was performed by the UW Biochemistry Department Sequencing Facility using primers for the T7 and SP6 regions of the vectors and sequence comparisons with public databases were performed via the Internet at the National Center for Biotechnology Information site (http://www.ncbi.nlm.nih.gov/), by employing the BLASTN algorithm (Altschul et al. 1997).
IAA production and root formation by endophytes
The sweet potato endophytic isolates were analyzed for IAA production. For rapid quantitative estimation in broth culture, the colorimetric method of Gordon and Weber (1951) was used. The cultures were grown in the dark for 7 d, sampled every day, centrifuged at 13000 x g for 10 min, and the production of IAA was assayed in duplicated supernatant samples. The presence of IAA in each supernatant was measured colorimetrically by adding two parts of 0.01 M FeCl3 in 35% HClO4 to one part of supernatant followed by reading the optical density at 530 nm after 25 min. The recorded absorbances were read off a standard curve prepared from pure IAA (Sigma-Aldrich, St. Louis, MO). The experiment was done in triplicate.
Another experiment was done to see if the IAA producing sweet potato endophytes can induce rooting in another plant species. We chose a hybrid poplar clone, INRA 717–1B4 (Populus tremula x P.alba) (Doty et al. 2007) which was difficult to root. Poplar cuttings about 8 cm were taken from the green house and surface sterilized using 10% bleach (0.615% NaClO) for 10 min and 1% iodophor for 5 min. The ends of the explants were removed and the stems were transferred to sterile culture tubes containing 10 ml of 1/2X Hoaglands solution (Hoagland and Arnon 1950) and grown for 2w. The most IAA producing endophyte, SPb (as determined from above) was streaked on an MG/L plate and grown overnight in MG/L broth. An inoculum of the endophyte (OD600 = 0.5) was added to five sterile tubes each containing MS growth media and one plant. Five uninoculated plants were also maintained and the tubes were incubated at 25°C for 3w with a 16 h photoperiod.
Stress tests
To determine if the sweet potato endophytes that produced high levels of IAA exhibited increased tolerance to stress, the endophytes were exposed to harsh conditions including heat and cold shock, UV irradiation, osmotic and heat shock, oxidative stress and antibiotic resistance. SPb, the most IAA producing endophyte was used for this study. SPh, that did not produce any IAA was used as a negative control. Cells were grown aerobically at 30°C in MG/L media. Maximum IAA production was observed in 2 days. At this point the cells were exposed to different stress tests. UV irradiation of cell suspensions (10 ml) was performed in the sterile hood exposing the cells to UV light for 2 h. For osmotic shock, the cells were incubated with 3 M sodium chloride for up to 2 h at 30°C. For acid stress, cells were grown at pH 3 and 4 for 2 h at 30°C. For oxidative stress, cells were treated with 3% H2O2 and grown for 2 h at 30°C. For heat shock cells were exposed to 75°C for 5 min by immersion of cultures in a water bath. For cold treatment, diluted cultures were plated on MG/L agar plates, and the plates were sealed in plastic bags to prevent drying, and incubated at 4°C for 1w. For antibiotic resistance the cultures were plated on MG/L amended with one of the following antibiotics (μg/ml): timentin (Tm) (250) (GlaxoSmithKline,NC), kanamycin (Km) (100) (plantMedia,Dublin,OH), ampicillin (Ap) (100) (Sigma,St.Louis,MO), chloramphenicol (Cm) (100) (Sigma,St.Louis,MO), cefatoxime (Cf) (500) (Aventis Pharmaceuticals, NJ), carbenicillin (Cb) 100 (Sigma,St.Louis,MO), and vancomycin (Vm) (30) (Abraxis, Schaumburg, IL). Growth was scored after overnight incubation of plates at 30°C.
Results
Identification
Eleven culturable bacterial endophytes were isolated from four sweet potato plants and characterized by comparative sequence analysis of the 16S rRNA region generated by PCR. The endophytes designated as SPa-SPk grew in 24 h except for SPg which took 2 days to form colonies. Distinct round colonies of sizes ranging from 1–3 mm and variable pigmentation (white, pink, and pale to bright yellow) were observed (Table 1). The 16S rRNA genes from most of the isolates possessed 99–100% similarity with a species already described in the GenBank. All of the strains belonged to the phylum proteobacteria, class- gammabacteria with the exception of Phyllobacterium that belongs to the alphaproteobacteria. A phylogenetic tree based upon nucleotide substitution values is depicted in Fig. 1.
Growth on nitrogen free medium and identification of the nitrogenase genes
As a first screen for the ability to fix nitrogen, the isolated sweet potato endophytes were streaked onto nitrogen-free medium plates with either glucose or sucrose as the carbon source. Only SPa grew when the carbon source was either sucrose or glucose; SPb grew only in the presence of sucrose. The growth curve experiments (Fig. 2) showed that SPa and SPb grew well in nitrogen free media. Statistical analysis of the data based on the primary analysis on log transformed absorbance gave strong evidence that the groups do differ between endophytes over time, with p < 0.0001.
Growth of sweet potato endophytes in nitrogen free medium. Azotobacter vinelandii (AZ) was used as a positive control. Agrobacterium tumefaciens (AG), which is a plant associated bacterium was used as negative control. SPa grew the best followed by SPb. The experiments were performed in duplicate and the bars indicate standard deviations
The nested PCR approach to identify the presence of nifH genes resulted in an expected amplicon for isolate Spa (approx. 371 bp). This PCR product was purified, subcloned and sequenced. The nifH sequence of SPa was most closely related to Azotobacter vinelandii (99% identity). The “universal” nifH primers only amplified a faint band of the expected size in isolate SPb (Fig. 3). Non-specific bands were also noticed, but after being cloned and sequenced they yielded no significant match to any known sequences in the databases.
Nested PCR amplification of nifH gene from SPa (lane 3) and SPb (lane 2) endophytes. The molecular weight standard was a 100 bp ladder (lane 1). A strong band at 371 bp for Spa confirmed the presence of nitrogenase gene. A faint band that could not be sequenced was observed for SPb at the expected size. Other non-specific bands were also obtained which DNA sequences did not match any known sequences. Azotobacter vinelandii DNA served as a positive control (lane 4) and for negative control we used water (lane 5) instead of DNA
IAA production and root induction
Four out of the eleven endophytes produced IAA (Fig. 4). Measurements of IAA of the four IAA-producing endophytes were significantly different (P < 0.05) from the other seven endophytes, which did not produce any IAA. To test if the endophytes of the sweet potato plant were helping it to grow, we propagated endophyte free plants by growing them in media containing antibiotics. These plants grew poorly suggesting the role of endophytes in plant growth. Also, when the maximum IAA producing endophyte (SPb) was inoculated into a hybrid poplar (Populus tremula x P.alba), INRA 717–1B4, which is normally difficult to root, excellent growth was observed. After 3 weeks, four out of the five endophyte-inoculated plants grew 5 cm taller, and had highly branched root systems and several more leaves than the uninoculated controls (Fig. 5).
Production of IAA by sweet potato endophytes. IAA reacts with FeCl3 and HClO4, and the absorbance of the color produced was measured at 530 nm on a spectrophotometer. SPb produced the maximum IAA followed by SPe, SPi and SPj. The experiment was performed in triplicate and the bars indicate standard deviations
Stress tests
It has been reported that higher amounts of IAA resulted in increased tolerance to stress, more active bacterial metabolism (Bianco et al. 2006; de Melo et al. 2004) and enhanced plant growth (Camerini et al. 2008). To determine if the sweet potato endophytes also had this characteristic, we chose SPb (maximum IAA producer) and SPh, that did not produce any IAA and was used as a negative control. Isolate SPb grew well in the presence of antibiotics including ampicillin, chloramphenicol, carbenicillin and vancomycin whereas no growth was observed when cefatoxime, timentin and kanamycin were present (Table 2). SPh did not tolerate any of the tested antibiotics. Cold and heat shock are physical stresses that drastically modify all physico-chemical parameters of a living cell. To test whether IAA production affected cell viability at temperatures that normally prevent growth, diluted cultures were plated on agar plates and inoculated at different temperatures. Isolate SPb survived cold treatment whereas SPh did not grow. The UV survival analysis showed that the IAA producing endophyte was more resistant (more CFU) to UV irradiation compared to the negative control. Finally, responses to heat shock, low pH, osmolarity and oxidative damage were similar (Table 2).
Discussion
Endophytes isolated from four sweet potato plants exhibited a wide range of phenotypic diversity and important functions. Growth in nitrogen free media, and the presence of nifH sequences verified in one of the isolate supports the hypothesis that the sweet potato endophytes may be of diazotrophic nature. Further studies are needed to determine if the endophytes might contribute to the N input in sweet potato plants that grow well in unfertile soil. Previous reports indicated that associative N2 fixation contributes to the N uptake in sweet potato plants (Yoneyama et al. 1998; Hill et al. 1990). Nitrogen fixing endophytes like Azospirillum sp. (Hill et al. 1983) and Gluconacetobacter diazotrophicus strains (Paula et al. 1991) were isolated from root and aerial part of sweet potato plants, respectively. Reiter et al. (2003) identified potential nitrogen fixing endophytes in seven sweet potato varieties collected in Uganda and Kenya. The nifH gene sequences had high homologies to the nitrogenase reductases of known nitrogen fixing bacteria. The authors suggested that the presence of these bacteria might contribute to the N input in sweet potato plants grown in different soils on small scale farms in Africa where no fertilizer is applied. In our study since Spa showed the presence of nitrogenase gene, we are currently doing experiments to investigate if it could help the sweet potato plant to grow in nitrogen limited conditions.
Some of the isolated endophytes of sweet potato synthesized the phytohormone IAA, which is involved in plant stem and root growth regulation. It is the most potent native auxin and is synthesized by plants from the amino acid tryptophan. The capacity to synthesize IAA production is widespread among soil and plant associated bacteria (Verma et al. 2001). Microorganisms including rhizobia, mycorrhizal fungi and pseudomonads which inhabit the aerial or subterranean surfaces of plants have been shown to be capable of IAA synthesis. The microbial synthesis of plant growth regulators is an important factor in soil fertility (Kampert and Strzelczyk 1975). Our results support that IAA production by some sweet potato endophytes could influence the host growth in low fertile soils. It has also been shown that bacteria that promote plant growth in a given plant species may have no effect or even inhibit the growth of another one (Lazarovits and Nowak 1997). In our study the most IAA producing sweet potato endophyte showed a plant growth promoting effect when inoculated into another plant. Cuttings of INRA 717–1B4 do not root when exogeneous auxin is provided, so perhaps the endophytes provide more than just the hormone improving the nutritional status of plants. Growth promoting effects by endophytic bacteria can also be cultivar specific (Bensalim et al. 1998). Further tests are underway to check if the IAA producing sweet potato endophytes are nonspecific.
Studies have shown that IAA triggers an increased level of protection against external adverse conditions by coordinately enhancing different cellular defence systems (Bianco et al. 2006). These authors investigated the effect of IAA treatment on bacterial cells and demonstrated that the cells were tolerant to a variety of stress conditions. This is in contrast to our findings where we observed that the IAA producer (SPb) and the non-producer (SPh) were both resistant to low pH, heat, osmotic and oxidative treatments. However, SPb was tolerant to certain antibiotics, UV irradiation and cold stress. Since no correlation between IAA production and stress response was found, other IAA-producing endophytes were not further tested. We speculate that the endophytes might be expressing certain stress proteins which assist in refolding of denatured proteins to help protect themselves from adverse conditions. Protective effects of endophytes on the plants and their survival in adverse environmental conditions have also been shown (Mattos et al. 2001). Endophytes produce proteins and enzymes with important biological functions that help in plant development (Sahai and Manocha 1993). Pleban et al. (1997) isolated endophytic B.cereus from mustard that produced an enzyme stable between pH 4 and 8.5 and significantly protected cotton seedlings from root rot disease caused by Rhizoctonia solani. Paenibacillus amylolyticus, an endophytic isolate from coffee cherries possessed thermo stable properties and pH stability and its bioactivity was hardly influenced by EDTA and any metal ion (Reinhold-Hurk and Hurek 1998; Sakiyama et al. 2001). Other reports have indicated that bacterial endophytes provide nutrient and mineral input to the plants (Kim et al. 2002; Pacovsky 1988; Malinowski et al. 2000), reduce heavy metal contamination (Malinowski et al. 2004), ameliorate disease development (Benhamou et al. 1996) and help acclimatize plants to environment stresses (Lazarovits and Nowak 1997). Thus endophytes endow their host plants with many benefits and their commercial potential could reasonably receive more attention.
A diverse array of bacterial species have been reported to be endophytic including Acetobacter, Arthrobacter, Bacillus, Burkholderia, Enterobacter, Herbaspirillum and Pseudomonas (Lodewyckz et al. 2002). The 16S rRNA of isolate SPb showed close relatedness to Rahnella aquatilis. Literature shows that Rahnella aquatilis is a plant associated bacterium and a nitrogen fixer. It was isolated from rhizosphere of wheat and maize and had the ability to reduce acetylene (indication of nitrogenase activity) in pure culture and in association with the host plant (Berge et al. 1991). Rahnella and Pseudomonas were identified as endophytes found inside seeds of Norway spruce where they were associated with plant growth promoting activity and biological control potential (Katarina et al. 2005).
The 16S rRNA gene sequence of SPe was closely related to that of Pseudomonas (100% identity). Beneficial Pseudomonas strains are frequently found associated with plants where they act as Plant Growth Promoting Bacteria (PGPB) by suppressing growth of pathogens or by producing plant growth hormones. Zaidi (2003) showed that when seeds of soybean were inoculated with a strain of Pseudomonas that produced IAA, the plants increased seed emergence. They also had increased shoot and root length, increased dry weight, increased numbers of nodules, and improved nutrient uptake. The bacteria were also resistant to several antibiotics.
There were several other isolates from sweet potato (SPh, SPi, SPj) that matched Enterobacter species. Enterobacteria, the most frequently occurring genera reported as endophytes are found in soil, water, fruits, vegetables, grains, flowering plants, and trees, and can be plant pathogens (Lodewyckz et al. 2002). Species of E. cloacae have been identified as endophytes of rice plants and had the ability to fix nitrogen (Ladha et al. 1983) and promote plant growth (Nie et al. 2002).
The sequence of 16S rRNA fragments of Spa and SPd had 100% identity with Stenotrophomonas maltophilia. Strains of this species have been isolated from RDX (Hexahydro-1,3,5-trinitro-1,3,5-triazine) and PAH (Polyaromatic hydrocarbon) contaminated soils (Binks et al. 1995; Boonchan et al. 1998). Some of the strains have been found to be multidrug resistant (Garrison et al. 1996), and colonizers of plant rhizosphere (Berg et al. 2005). This genus was among several other bacteria isolated as an endophyte in coffee seeds (Vega et al. 2005). Lata et al. (2006) found endophytic bacteria belonging to Pseudomonas and Stenotrophomonas associated with Echinacea plants.
Isolates SPf and SPk matched Xanthomonas sp. Xanthomonas is a genus of proteobacteria, many of which cause plant diseases. Some strains have always been found in association with plants. Tanprasert and Reed (1997) identified these as endophytes isolated from strawberry.
The sequence of the 16S rRNA fragment of SPg had 100% identity with Rhodanobacter species. Strains of this group have been isolated from soil (Weon et al. 2007) and were found to degrade carcinogens (Kanaly et al. 2002) and have biocontrol activity (Clercq et al. 2006). The presence of an endophyte that resembles a strain that degrades carcinogens is an encouraging result since it has been shown that several endophytic strains present in different plants have the potential to degrade pollutants.
The 16S rRNA sequence of sweet potato endophyte, SPc, had 100% identity with Phyllobacterium sp. which is a plant growth promoting bacteria (Mantelin et al. 2006; Bertrand et al. 2001) able to fix nitrogen (Lambert and Joos 1989). It has been isolated from the rhizosphere of mangroves (Adriana et al. 2001) and sugar beet plants (Lambert et al. 1990). It has also been found to increase availability of nutrients in the rhizosphere positively influencing root growth and morphology and promoting other plant microbe symbioses (Vessey 2003).
Since sweet potato plants are hardy, fast growing, have an extensive root system, grow in poor soils and have a wide geographical distribution, they can be well suited for phytoremediation applications to clean up land contaminated with harmful pollutants. Also this starch rich root crop is a potential biofuel source and the tubers can be harvested after phytoremediation is complete to make liquid biofuels. This preliminary study yielded important uses of the endophytes to the sweet potato plants. We are currently investigating if any of these endophytes can aid in phytoremediation of carcinogenic compounds as well as help in plant development under different environmental stresses (temperature, osmosis, dessication).
References
Adriana R, Holguin G, Glick BR et al (2001) Synergism between Phyllobacterium sp. (N2-fixer) and Bacillus licheniformis (P-solubilizer), both from a semiarid mangrove rhizosphere. FEMS Microbiol Ecol 35(2):181–187. doi:10.1111/j.1574-6941.2001.tb00802.x
Altschul SF, Madden TL, Schaffer AA et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi:10.1093/nar/25.17.3389
Ausubel F, Brent R, Kingston RE et al (1995) Short protocols in molecular biology. E d. John Wiley & Sons, Inc, New York
Bangera MG, Thomashow LS (1996) Characterization of a genomic locus required for synthesis of the antibiotic 2,4-diacetylphloroglucinol by the biological control agent Pseudomonas fluorescens Q2–87. Mol Plant Microbe Interact 9:83–90
Barraquio WL, Revilla L, Ladha JK (1997) Isolation of endophytic diazotrophic bacteria from wetland rice. Plant Soil 194:15–24. doi:10.1023/A:1004246904803
Benhamou N, Kloepper JW, Quadt-Hallman A et al (1996) Induction of defence related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol 112:919–929
Bensalim S, Nowak J, Asiedu S (1998) A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am J Potato Res 75:145–152
Berg G, Eberl L, Hartmann A (2005) The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol 7:1673–1685. doi:10.1111/j.1462-2920.2005.00891.x
Berge O, Heulin T, Achouak W et al (1991) Rahnella aquatilis, a nitrogen fixing enteric bacterium associated with the rhizosphere of wheat and maize. Can J Microbiol 37:195–203
Bertrand H, Nalin R, Bally R et al (2001) Isolation and identification of the most efficient plant growth promoting bacteria associated with canola (Brassica napus). Biol Fertil Soils 33:152–156. doi:10.1007/s003740000305
Bianco C, Imperlini E, Calogero R et al (2006) Indole 3-acetic acid improves Escherichia coli’s defences to stress. Arch Microbiol 185:373–382. doi:10.1007/s00203-006-0103-y
Binks PR, Nicklin S, Bruce N (1995) Degradation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) by Stenotrophomonas maltophilia PB1. Appl Environ Microbiol 61:1318–1322
Boonchan S, Britz ML, Stanley GA (1998) Surfactant enhanced biodegradation of high molecular weight PAHs by Stenotrophomonas maltophilia. Biotechnol Bioeng 59:482–494. doi:10.1002/(SICI)1097-–0290(19980820)59:4<482::AID--BIT11>3.0.CO;2--C
Burgmann H, Widmer F, Sigler WV et al (2004) New molecular screening tools for the analysis of free living diazotrophs in soil. Appl Environ Microbiol 70:240–247. doi:10.1128/AEM.70.1.240–247.2004
Camerini S, Senatore B, Lonardo E et al (2008) Introduction of a novel pathway for IAA biosynthesis to rhizobia alters vetch root nodule development. Arch Microbiol 190:67–77. doi:10.1007/s00203-008-0365-7
Chilton MD, Currier T, Farrand S et al (1974) Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA 71:3672–3676. doi:10.1073/pnas.71.9.3672
Clercq D, Trappen SV, Cleenwerck I et al (2006) Rhodanobacter spathiphylli sp.nov., a gammaproteobacterium isolated from the roots of Spathiphyllum plants grown in a compost amended potting mix. Int J Syst Evol Microbiol 56:1755–1759. doi:10.1099/ijs.0.64131-0
Creus CM, Sueldo RJ, Barassi C (1998) Water relations in Azospirillum inoculated wheat seedlings under osmotic stress. Can J Bot 76:238–244. doi:10.1139/cjb-76-2-238
deAraujo BS, de Oliveira JO, Machado SS et al (2004) Comparative studies of the peroxidases from hairy roots of Daucus carota, Ipomoea batatas and Solanum aviculare. Plant Sci 167:1151–1157
de Melo MP, Pithon-Curi TC, Curi R (2004) Indole-3-acetic acid increases glutamine utilization by high peroxidase activity-presenting leucocytes. Life Sci 75:1713–1725
Doty SL, Dosher MR, Singleton GL et al (2005) Identification of an endophytic rhizobium in stems of Populus. Symbiosis 39:27–35
Doty SL, James CA, Moore AL et al (2007) Enhanced phytoremediation of volatile environmental pollutants with transgenic trees. Proc Natl Acad Sci 104:16816–16821
Doty SL (2008) Tansley review: enhancing phytoremediation through the use of transgenics and endophytes. New Phytol 179:318–333. doi:10.1111/j.1469-8137.2008.02446.x
Frommel MI, Nowak J, Lazarovits G (1991) Growth enhancement and developmental modifications of in vitro grown potato (Solanum tuberosum sp. tuberosum) as affected by a nonfluorescent Pseudomonas sp. Plant Physiol 96:928–936
Garrison MW, Anderson DE, Campbell DM et al (1996) Stenotrophomonas maltophilia: emergence of multidrug resistant strains during therapy in an in vitro pharmacodynamic chamber model. Antimicrobial agents and Chemotherapy 40:2859–2864
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J Theor Biol 190:63–68
Gordon SA, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26:192–195
Hallmann J, Quadt-Hallmann A, Mahaffee WF et al (1997) Endophytic bacteria in agricultural crops. Can J Microbiol 43:895–914
Hill WA, Bacon Hill P, Crossman SM et al (1983) Characterisation of N2-fixing bacteria associated with sweet potato roots. Can J Microbiol 29:860–862
Hill WA, Dodo H, Hahn SK et al (1990) Sweet potato root and biomass production with and without fertilization. Agron J 82:1120–1122
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Circular 347, Calif Agric Expt Stn, University of California, Berkeley, CA
Jacobson CB, Pasternak JJ, Glick BR (1994) Partial purification and characterization of 1-amino-cyclopropane-1-carboxylate deaminase from the plant growth promoting rhizobacterium Pseudomonas putida GR 12–2. Can J Microbiol 40:1019–1025
Kampert M, Strzelczyk E (1975) Synthesis of auxins by fungi isolated from the roots of pine seedlings (Pinus silvestries L.) and from soil. Acta Microbiol Paleon Ser B 7:223–230
Kanaly RA, Harayama S, Watanabe K (2002) Rhodanobacter sp. strain BPC1 in a Benzo(a)pyrene mineralizing bacterial consortium. Appl Environ Microbiol 68:5826–5833
Katarina C, Hojka K, Ravnikar M et al (2005) Bacterial endophytes from seeds of Norway spruce (Picea abies L. Karst). FEMS Microbiol Letters 244:341–345
Kim KY, Cho YS, Sohn BK et al (2002) Cold storage of mixed inoculum of Glomus intraradius enhances root colonization, phosphorous status and growth of hot pepper. Plant Soil 238:267–272
Ladha JK, Barraquio WL, Watanabe I (1983) Isolation and identification of nitrogen fixing Enterobacter cloacae and Klebsiella planticola associated with rice plants. Can J Microbiol 29:1301–1308
Lambert B, Joos H (1989) Fundamental aspects of rhizobacterial plant growth promotion research. Trends Biotechnol 7:215–219
Lambert B, Joos H, Dierickx S et al (1990) Identification and plant interaction of a Phyllobacterium sp., a predominant rhizobacterium of young sugar beet plants. Appl Environ Microbiol 56:1093–1102
Lata H, Li XC, Silva B et al (2006) Identification of IAA producing endophytic bacteria from micropropagated echinacea plants using 16S rRNA sequencing. Plant Cell Tissue Organ Cult 85:353–359
Lazarovits G, Nowak J (1997) Rhizobacteria for improvement of plant growth and establishment. HortScience 32:188–192
Lodewyckz C, Vangronsveld J, Porteous F et al (2002) Endophytic bacteria and their potential applications. Crit Rev Plant Sci 21:583–606
Madhaiyan M, Poonguzhali S, Senthilkumar M et al (2004) Growth promotion and induction of systemic resistance in rice cultivar Co-47 (Oryza sativa L.) by Methylobacterium sp. Bot Bull Acad Sin 45:315–324
Malinowski DP, Alloush GA, Belesky DP (2000) Leaf endophyte Neotyphodium coenophialum modifies mineral uptake in tall fescue. Plant Soil 227:115–126
Malinowski DP, Zuo H, Belesky DP et al (2004) Evidence of copper binding by extracellular root exudates of tall fescue but not perennial ryegrass infected with Neotyphodium sp. endophytes. Plant Soil 267:1–12
Mantelin S, Desbrosses G, Larcher M et al (2006) Nitrate-dependent control of root architecture and N nutrition are altered by a plant growth-promoting Phyllobacterium sp. Planta 223:591–603
Mattos KA, Jones C, Heise N et al (2001) Structure of an acidic exopolysachharide produced by the diazotrophic endophytic bacterium Burkholderia brasiliensi. Eur J Biochem 268:3174–3179
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Plant Physiol 15:473–497
Nie L, Shah S, Rashid A et al (2002) Phytoremediation of arsenate contaminated soil by transgenic canola and the plant growth promoting bacterium Enterobacter cloacae CAL2. Plant Physiol Biochem 40:355–361
Nowak J, Asiedu SK, Lazarovits G (1995) Enhancement of in vitro growth and transplant stress tolerance of potato and vegetable plantlets co-cultured with a plant growth promoting pseudomonad bacterium. In: Carre F, Chagvardieff P (eds) Proc. International Symposium on Ecophysiology and Photosynthetic in Vitro Cultures, Aix-en-Provence, France CEA, Cadarache, France, pp173–180
O Sullivan DJ, O, Gara F (1992) Traits of fluorescent Pseudomonas sp. involved in suppression of plant root pathogens. Microbial Rev 56:662–676
Pacovsky RS (1988) Influence of inoculation with Azospirillum brasilense and Glomus fasciculatum on sorghum nutrition. Plant Soil 110:283–287
Paula MA, Reis UM, Dobereiner J (1991) Interactions of Golum clarum with Acetobacter diazotropicus in infection of sweet potato, sugarcane and sweet sorghum. Biol Fertil Soils 11:111–115
Pleban S, Chernin L, Chet I (1997) Chitinolytic activity of an endophytic strain of Bacillus cereus. Lett Appl Microbiol 25:284–288
Redman RS, Sheehan KB, Stout RG et al (2002) Thermotolerance generated by plant/fungal symbiosis. Science 298:1581
Reinhold-Hurk B, Hurek T (1998) Life in grasses:diazotrophic endophytes. Trends Microbiol 6:139–144
Reiter B, Burgmann H, Burg K et al (2003) Endophytic gene diversity in African sweet potato. Can J Microbiol 49:549–555
Sahai AS, Manocha MS (1993) Chitinases of fungi and plants:their involvement in morphogenesis and host-parasite interaction. FEMS Microbiol Rev 11:317–338
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sakiyama CCH, Paula EM, Pereira PC et al (2001) Characterization of pectin lyase produced by an endophytic strain isolated from coffee cherries. Lett Appl Microbiol 33:117–121
Strobel G, Daisy B, Castillo U et al (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Tamura K, Dudley J, Nei M et al (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tanprasert P, Reed BM (1997) Detection and identification of bacterial contaminants from strawberry runner explants. In Vitro Cell Dev Biol 33:221–226
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Ulrich K, Ulrich A, Ewald D (2008) Diversity of endophytic bacterial communities in poplar grown under field conditions. FEMS Microbiol Ecol 63:169–180
Vega FE, Ripoll MP, Posada F et al (2005) Endophytic bacteria in Coffea arabica L. J Basic Microbiol 45:371–380
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotech 91:127–141
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Weon HY, Kim BY, Hong SB et al (2007) Rhodanobacter ginsengisoli sp. nov and Rhodanobacter terrae sp. nov. isolated from soil cultivated with Korean ginseng. Int J Syst Evol Microbiol 57:2810–2813
Yoneyama T, Terakado J, Masuda T (1998) Natural abundance of 15N in sweet potato, pumpkin, sorghum and castor bean: possible input of N2 derived nitrogen in sweet potato. Biol Fertil Soils 26:152–154
Zaidi SFA (2003) Biocontrol of Fusarium oxysporium by plant growth promoting rhizobacteria in soybean. Ann Agr Res 24:676–678
Acknowledgements
We thank undergraduate student helper Amanda Thornton for helping with the isolation of endophytes and the United States Department of Agriculture (USDA) for funding this project (grant no: 58-3148-5159)
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jesus Mercado-Blanco.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Khan, Z., Doty, S.L. Characterization of bacterial endophytes of sweet potato plants. Plant Soil 322, 197–207 (2009). https://doi.org/10.1007/s11104-009-9908-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-9908-1