Abstract
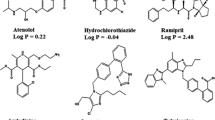
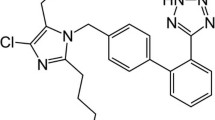
A simple, rapid, and precise reversed-phase high-performance liquid chromatographic method has been developed for simultaneous determination of losartan potassium, ramipril, and hydrochlorothiazide. The three drugs were separated on a 150 mm × 4.6 mm i.d., 5 μm particle, Cosmosil C18 column. The mobile phase was 0.025 m sodium perchlorate–acetonitrile, 62:38 (v/v), containing 0.1% heptanesulphonic acid, pH adjusted to 2.85 with orthophosphoric acid, at a flow rate of 1.0 mL min−1. UV detection was performed at 215 nm. The method was validated for linearity, accuracy, precision, and limit of quantitation. Linearity, accuracy, and precision were acceptable in the ranges 35–65 μg mL−1 for losartan, 1.75–3.25 μg mL−1 for ramipril, and 8.75–16.25 μg mL−1 for hydrochlorothiazide.




Similar content being viewed by others
References
Merck Index (2001) 13th edn. Merck & Co., inc., USA
Kanumula GV, Bhanu R (2000) Indian Drugs 37:38–41
Zarapkar SS, Rane SH (2000) Indian Drugs 37:589–593
Shah SA, Rathod IS, Suhagia BN, Salve SS, Patel JB (2001) J AOAC Int 84:1715–1723
Hertzog DL, McCafferty JF, Fang X, Tyrrell RJ, Reed RA (2002) J Pharm Biomed Anal 30:747–760
Gandhimathi M, Ravi TK, Ninan A, Varghese A (2004) Indian Drugs 41:36–39
Valiyare GR, Chandra A, Apte SK, Mahadik AA (2005) Indian Drugs 42:309–312
Snyder LR, Kirkland JJ, Glajch JL (1997) Practical HPLC method development, 2nd edn. Wiley, USA
ICH (1996) Validation of analytical procedures: methodology, ICH harmonised tripartite guidelines, adopted 6 Nov 1996
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baing, M.M., Vaidya, V.V., Sane, R.T. et al. Simultaneous RP-LC Determination of Losartan Potassium, Ramipril, and Hydrochlorothiazide in Pharmaceutical Preparations. Chroma 64, 293–296 (2006). https://doi.org/10.1365/s10337-006-0008-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-006-0008-6