Abstract
Background
The optimal treatment strategy for small node-negative human epidermal growth factor receptor 2-positive (HER2+) breast cancer remains controversial. Neoadjuvant chemotherapy may risk overtreatment, whereas surgery first fails to identify patients with residual disease in need of escalated adjuvant systemic therapy. We investigated patient characteristics associated with receipt of neoadjuvant chemotherapy.
Methods
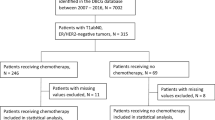
Adult women with cT1-T2/N0, HER2+ breast cancer between 2013 and 2017 in the National Cancer Database who underwent surgery within 8 months of diagnosis were included. Patients were classified as receiving neoadjuvant chemotherapy versus a surgery-first approach. We assessed the sociodemographic and clinical predictors of neoadjuvant chemotherapy versus surgery first and associations between neoadjuvant chemotherapy and breast cancer treatments using multivariable regression models.
Results
We identified 56,784 women, of whom 12,758 (22%) received neoadjuvant chemotherapy, 29,139 (53%) received adjuvant chemotherapy, 12,907 (24%) received no chemotherapy, and 1980 were missing chemotherapy information. After adjustment, cT2 stage was the strongest predictor of neoadjuvant chemotherapy compared with surgery first. Younger age and later diagnosis year were positively associated with receipt of neoadjuvant chemotherapy. In contrast, hormone receptor positivity, Black race, rural county, and government-funded or no health insurance were inversely associated with neoadjuvant chemotherapy. In multivariable analyses, patients who received neoadjuvant chemotherapy were more likely to have a mastectomy (vs. lumpectomy) and sentinel lymph node biopsy or no nodal surgery (vs. axillary lymph node dissection). Patients who received neoadjuvant chemotherapy were more likely to receive multi-agent (vs. single-agent) chemotherapy than those who received adjuvant chemotherapy.
Conclusions
Substantial differences in the utilization of neoadjuvant chemotherapy exist in women with HER2+ breast cancer, which reflect both clinical parameters and disparities. Optimal treatment strategies should be implemented equitably across sociodemographic groups.



Similar content being viewed by others
References
National Cancer Institute. Surveillance Epidemiology and End Results (SEER) program. Cancer Stat Facts: Female Breast Cancer. 2021. Available at: https://seer.cancer.gov/statfacts/html/breast.html. Accessed 11 May 2021.
Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–29.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72.
Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83.
von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28.
Choong GM, Cullen GD, O’Sullivan CC. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA Cancer J Clin. 2020;70(5):355–74.
File D, Curigliano G, Carey LA. Escalating and de-escalating therapy for early-stage HER2-positive breast cancer. Am Soc Clin Oncol Educ Book. 2020;40:1–11.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Version 4.2021. 28 April 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 11 May 2021.
de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137–46.
Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–7.
Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134–41.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–90.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Sturmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med. 2014;275(6):570–80.
Tolaney SM, Tayob N, Dang C, et al. Adjuvant trastuzumab emtansine versus paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT): a randomized clinical trial. J Clin Oncol. 2021;39(21):2375–85.
Giordano SH, Niu J, Chavez-MacGregor M, et al. Estimating regimen-specific costs of chemotherapy for breast cancer: observational cohort study. Cancer. 2016;122(22):3447–55.
Kale HP, Carroll NV. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122(8):283–9.
Magnuson A, Lei L, Gilmore N, et al. Longitudinal relationship between frailty and cognition in patients 50 years and older with breast cancer. J Am Geriatr Soc. 2019;67(5):928–36.
Mariano C, Lund JL, Peacock Hinton S, Htoo P, Muss H, Reeder-Hayes KE. Evaluating the association between adjuvant chemotherapy and function-related adverse events among older patients with early stage breast cancer. J Geriatr Oncol. 2017;8(4):242–8.
Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41(17):2613–9.
Freedman RA, Hughes ME, Ottesen RA, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119(4):839–46.
Mujahid MS, Janz NK, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. The impact of sociodemographic, treatment, and work support on missed work after breast cancer diagnosis. Breast Cancer Res Treat. 2010;119(1):213–20.
Zeidman M, Alberty-Oller JJ, Ru M, et al. Use of neoadjuvant versus adjuvant chemotherapy for hormone receptor-positive breast cancer: a National Cancer Database (NCDB) study. Breast Cancer Res Treat. 2020;184(1):203–12.
Valachis A, Mauri D, Polyzos NP, Chlouverakis G, Mavroudis D, Georgoulias V. Trastuzumab combined to neoadjuvant chemotherapy in patients with HER2-positive breast cancer: a systematic review and meta-analysis. Breast. 2011;20(6):485–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Emilie Duchesneau is supported by the Cancer Care Quality Training Program at the University of North Carolina at Chapel Hill (Grant T32CA116339); Stephanie Downs-Canner is supported by the National Institutes of Health (Grant 5K12CA120780-12; Philip Spanheimer is supported by the National Institutes of Health (Grant P50CA058223); and Paula D. Strassle is supported by the Division of Intramural Research, National Institute on Minority Health and Health Disparities, National Institutes of Health. Selena J. An, Katherine Reeder-Hayes, Kristalyn K. Gallagher, and David W. Ollila have no disclosures to declare. This work was supported in part by P30 CA016086 UNC Lineberger Cancer Center Core Support Grant. The contents and views in this manuscript are those of the authors and should not be construed to represent the views of the National Institutes of Health.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duchesneau, E.D., An, S.J., Strassle, P.D. et al. Sociodemographic and Clinical Predictors of Neoadjuvant Chemotherapy in cT1-T2/N0 HER2-Amplified Breast Cancer. Ann Surg Oncol 29, 3051–3061 (2022). https://doi.org/10.1245/s10434-021-11260-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11260-y