Abstract
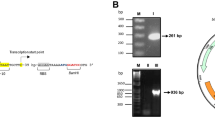
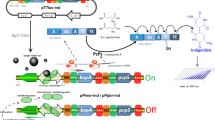
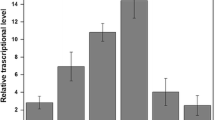
Aniline and chlorinated anilines (CAs) are classified as priority pollutants; therefore, an effective method for detection and monitoring is required. In this study, a green-fluorescence protein-based bioreporter for the detection of aniline and CAs was constructed in Escherichia coli DH5α, characterized and tested with soil and wastewater. The sensing capability relied on the regulatory control between a two-component regulatory protein, TodS/TodT, and the P todX promoter of Pseudomonas putida T-57 (PpT57), since the gene expression of todS, todT, and todC2 are positively induced with 4-chloroaniline. The bioreporter system (DH5α/pPXGFP–pTODST) is markedly unique with the two co-existing plasmids. The inducibility of the fluorescence response was culture-medium- and time-dependent. Cells grown in M9G medium exhibited a low background fluorescence level and were readily induced by 4CA after 3-h exposure, reaching the maximum induction level at 9 h. When tested with benzene, toluene, ethyl-benzene and xylene, aniline and CAs, the response data were best fit by a sigmoidal dose–response relationship, from which the K 1/2 value was determined for the positive effectors. 3CA and 4CA were relatively powerful inducers, while some poly-chlorinated anilines could also induce green fluorescence protein expression. The results indicated a broader recognition range of PpT57’sTodST than previously reported for P. putida. The test results with environmental samples were reliable, indicating the potential application of this bioreporter in the ecotoxicology assessment and bioremediation of areas contaminated with aniline- and/or CAs.




Similar content being viewed by others
References
Applegate B, Kelly C, Lackey L, McPherson J, Kehrmeyer S, Menn FM, Bienkowski P, Sayler G (1997) Pseudomonas putida B2: a tod-lux bioluminescent reporter for toluene and trichloroethylene co-metabolism. J Ind Microbiol Biotechnol 18(1):4–9
Applegate BM, Kehrmeyer SR, Sayler GS (1998) A chromosomally based tod-luxCDABE whole-cell reporter for benzene, toluene, ethylbenzene, and xylene (BTEX) sensing. Appl Environ Microbiol 64(7):2730–2735
Behzadian F, Barjeste H, Hosseinkhani S, Zarei AR (2011) Construction and characterization of Escherichia coli whole-cell biosensors for toluene and related compounds. Curr Microbiol 62(2):690–696. doi:10.1007/s00284-010-9764-5
Burlage RS, Palumbo AV (1994) Bioluminescent reporter bacteria detect contaminants in soil samples. Appl Biochem Biotechnol 46:731–740
Busch A, Guazzaroni ME, Lacal J, Ramos JL, Krell T (2009) The sensor kinase TodS operates by a multiple step phosphorelay mechanism involving two autokinase domains. J Biol Chem 284(16):10353–10360. doi:10.1074/jbc.M900521200
Busch A, Lacal J, Martos A, Ramos JL, Krell T (2007) Bacterial sensor kinase TodS interacts with agonistic and antagonistic signals. Proc Natl Acad Sci USA 104(34):13774–13779. doi:10.1073/pnas.0701547104
Busch A, Lacal J, Silva-Jimenez H, Krell T, Ramos JL (2010) Catabolite repression of the TodS/TodT two-component system and effector-dependent transphosphorylation of TodT as the basis for toluene dioxygenase catabolic pathway control. J Bacteriol 192(16):4246–4250. doi:10.1128/JB.00379-10
de las Herasde A, Lorenzo V (2011) In situ detection of aromatic compounds with biosensor Pseudomonas putida cells preserved and delivered to soil in water-soluble gelatin capsules. Anal Bioanal Chem 400(4):1093–1104. doi:10.1007/s00216-010-4558-y
Dom N, Knapen D, Benoot D, Nobels I, Blust R (2010) Aquatic multi-species acute toxicity of (chlorinated) anilines: experimental versus predicted data. Chemosphere 81(2):177–186. doi:10.1016/j.chemosphere.2010.06.059
Faizal I, Dozen K, Hong CS, Kuroda A, Takiguchi N, Ohtake H, Takeda K, Tsunekawa H, Kato J (2005) Isolation and characterization of solvent-tolerant Pseudomonas putida strain T-57, and its application to biotransformation of toluene to cresol in a two-phase (organic-aqueous) system. J Ind Microbiol Biotechnol 32(11–12):542–547
Farinha MA, Kropinski AM (1990) Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol 172(6):3496–3499
Hansen LH, Sorensen SJ (2000) Versatile biosensor vectors for detection and quantification of mercury. FEMS Microbiol Lett 193(1):123–127
Harms H, Wells MC, van der Meer JR (2006) Whole-cell living biosensors–are they ready for environmental application? Appl Microbiol Biotechnol 70(3):273–280. doi:10.1007/s00253-006-0319-4
Keane A, Phoenix P, Ghoshal S, Lau PC (2002) Exposing culprit organic pollutants: a review. J Microbiol Methods 49(2):103–119
Lacal J, Busch A, Guazzaroni ME, Krell T, Ramos JL (2006) The TodS–TodT two-component regulatory system recognizes a wide range of effectors and works with DNA-bending proteins. Proc Natl Acad Sci USA 103(21):8191–8196. doi:10.1073/pnas.0602902103
Li YF, Li FY, Ho CL, Liao VH (2008) Construction and comparison of fluorescence and bioluminescence bacterial biosensors for the detection of bioavailable toluene and related compounds. Environ Pollut 152(1):123–129. doi:10.1016/j.envpol.2007.05.002
Moller S, Sternberg C, Andersen JB, Christensen BB, Ramos JL, Givskov M, Molin S (1998) In situ gene expression in mixed-culture biofilms: evidence of metabolic interactions between community members. Appl Environ Microbiol 64(2):721–732
Phoenix P, Keane A, Patel A, Bergeron H, Ghoshal S, Lau PC (2003) Characterization of a new solvent-responsive gene locus in Pseudomonas putida F1 and its functionalization as a versatile biosensor. Environ Microbiol 5(12):1309–1327
Robbens J, Dardenne F, Devriese L, De Coen W, Blust R (2010) Escherichia coli as a bioreporter in ecotoxicology. Appl Microbiol Biotechnol 88(5):1007–1025. doi:10.1007/s00253-010-2826-6
Sagi E, Hever N, Rosen R, Bartolome AJ, Premkumar JR, Ulber R, Lev O, Scheper T, Belkin S (2003) Fluorescence and bioluminescence reporter functions in genetically modified bacterial sensor strains. Sens Actuators B Chem 90:2–8
Tecon R, Beggah S, Czechowska K, Sentchilo V, Chronopoulou PM, McGenity TJ, van der Meer JR (2010) Development of a multi strain bacterial bioreporter platform for the monitoring of hydrocarbon contaminants in marine environments. Environ Sci Technol 44(3):1049–1055. doi:10.1021/es902849w
van der Meer JR (2010) Bacterial sensors: synthetic design and application principles. Morgan & Claypool publishers, Lausanne
Vangnai AS, Petchkroh W (2007) Biodegradation of 4-chloroaniline by bacteria enriched from soil. FEMS Microbiol Lett 268:209–216
Willardson BM, Wilkins JF, Rand TA, Schupp JM, Hill KK, Keim P, Jackson PJ (1998) Development and testing of a bacterial biosensor for toluene-based environmental contaminants. Appl Environ Microbiol 64(3):1006–1012
Acknowledgments
The financial support from the Environmental Research and Management Department, PTT Research and Technology Institute, and from the collaborative research project between Chulalongkorn University and Hiroshima University, is acknowledged. The authors gratefully acknowledge Dr. Robert Butcher, PCU, Faculty of Science, Chulalongkorn University, for his invaluable comments and review.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vangnai, A.S., Kataoka, N., Soonglerdsongpha, S. et al. Construction and application of an Escherichia coli bioreporter for aniline and chloroaniline detection. J Ind Microbiol Biotechnol 39, 1801–1810 (2012). https://doi.org/10.1007/s10295-012-1180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1180-3