Abstract
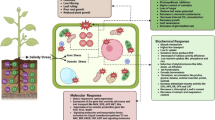
Nitric oxide (NO) is a highly reactive, membrane-permeable free radical, which has recently emerged as an important signalling molecule and antioxidant. Here we investigated the protective effect of NO against the toxicity caused by excess CuSO4 (50 μM) in the adventitious roots of mountain ginseng. It was found that NO donor, sodium nitroprusside (SNP), was effective in reducing Cu-induced toxicity in the mountain ginseng adventitious roots. Protective effect of SNP, as indicated by extent of lipid peroxidation, was reversed by incorporation of 2-(4-carboxy-2-phenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (CPTIO), a NO scavenger, in the medium suggesting that the protective effect of SNP is attributable to NO released, which was revealed from in situ confocal laser scanning microscopic localization of NO in the adventitious roots of mountain ginseng. Results obtained in the present study suggest that reduction of excess Cu-induced toxicity by SNP is most likely mediated through the modulation in the activities of antioxidant enzymes involved in H2O2 detoxification (catalase, peroxidase, ascorbate peroxidase) and in the maintenance of cellular redox couples (glutathione reductase), and contents of molecular antioxidants (particularly non-protein thiol, ascorbate and its redox status). Exogenous NO supply also improved the activity of superoxide dismutase, an enzyme responsible for O2 ·− dismutation, and NADPH oxidase, an enzyme responsible for O2 ·− generation, in excess Cu supplied adventitious roots of mountain ginseng.






Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- CPTIO:
-
2-(4-carboxy-2-phenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide
- EDTA:
-
Ethylenediamine tetraacetic acid
- GR:
-
Glutathione reductase
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SNP:
-
Sodium nitroprusside
- SOD:
-
Superoxide dismutase
- NO:
-
Nitric oxide
- TCA:
-
Trichloroacetic acid
References
Ali MB, Hahn EJ, Paek KY (2006) Copper-induced changes in the growth, oxidative metabolism and saponin production in suspension culture roots of Panax ginseng in bioreactors. Plant Cell Rep. 25:1122–1132
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–379
Arasimowicz M, Floryszak-Wieczorek J (2007). Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci 172:876–887
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tiss Org Cult 39:7–12
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beligni MV, Lamattina L (1999) Nitric oxide protects against cellular damage produced by methylviologen herbicides in potato plants. Nitric Oxide 3:199–208
Beligni MV, Fath A, Bethke PC, Lamattina L, Jones RL (2002) Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol 129:1642–1650
Bisht SS, Sharma A, Chaturvedi K (1989) Certain metabolic lesions of chromium toxicity in radish. Indian J Agric Biochem 2:109–115
Boveris AD, Galatro A, Puntarulo S. (2000) Effect of nitric oxide and plant antioxidants on microsomal content of lipid radicals. Biol Res 33:159–165
Brennan T, Frenkel C. (1977) Involvement of hydrogen peroxide in regulation of senescence in pear. Plant Physiol 59:411–416
Carimi F, Zottini M, Costa A, Cattelan I, Michele RD, Terzi M, Schiavo FL (2005) NO signalling in cytokinin-induced programmed cell death. Plant Cell Environ 28:1171–1178
Corpas FJ, Barroso JB, Carreras A, Quiros M, Leon AM, Romero-Puertas MC, Esteban FJ, Valderrama R, Palma JM, Sandalio LM, Gomez M, del Rio LA (2004) Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants. Plant Physiol 136:2722–2733
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Halliwell B. (2006) Reactive species and antioxidants. redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–14
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast, I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:180–198
Hsu YT, Kao CH (2004) Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227–38
Hung KT, Kao CH (2003) Nitric oxide counteracts the senescence of rice leaves induced by abscisic acid. J Plant Physiol 160:871–879
Hung KT, Kao CH (2004) Nitric oxide acts as an antioxidant and delays methyl jasmonate-induced senescence of rice leaves. J Plant Physiol 161:43–52
Hung KT, Chang CJ, Kao CH (2002). Paraquat toxicity is reduced by nitric oxide in rice leaves. J Plant Physiol 159:159–166
Jablonski PP, Anderson JW (1978) Light-dependent reduction of oxidised glutathione by ruptured chloroplasts. Plant Physiol 61:221–225
Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. (1998) Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70:2446–2453
Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330
Liszkay A, van der Zalm E, Schopfer P. (2004) Production of reactive oxygen intermediates O2 ·−, H2O2, and OH˙ by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Maksymiec W (1997) Effect of copper on cellular processes in higher plants. Photosynthetica 34:321–342
Maksymiec W, Krupa Z. (2006) The effects of short-term exposition to Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ Exp Bot. 57:187–194
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Murashige T., Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Murgia I, de Pinto MC, Delledonne M, Soave C, Gara LD (2004) Comparative effects of various nitric oxide donors on ferritin regulation, programmed cell death, and cell redox state in plant cells. J Plant Physiol 161:777–783
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Orozco-Cardenas M, Ryan CA (2002) Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol 130:487–93
Pätsikkä E, Kairavuo M, Šeršen F, Aro E-M, Tyystjärvi E. (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129:1359–1367
Quartacci MF, Cosi E, Navari-Izzo F (2001) Lipid and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J Exp Bot 52:77–84
Rao MV, Hale BA, Ormrod DP (1995) Amelioration of ozone-induced oxidative damage in wheat plants grown under high carbon dioxide. Plant Physiol 109:421–32
Rubbo H, Radi R, Anselmi D, Kirk M, Barnes S, Butler J, Eiserich JP, Freeman BA (2000) Nitric oxide reaction with lipid peroxyl radicals spares alpha-tocopherol during lipid peroxidation. Greater oxidant protection from the pair nitric oxide/alphatocopherol than alpha-tocopherol/ascorbate. J Biol Chem 275:10812–10818
Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91(phox) NADPH oxidase: modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126:1281–1290
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340
Sarath G, Hou G, Baird LM, Mitchell RB (2007) Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C4-grasses. Planta doi 10.1007/s00425-007-0517-z
Sharma SS, Dietz KJ, (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress J Exp Bot 57:711–726
Singh AK, Sharma L, Mallick N (2004) Antioxidative role of nitric oxide on copper toxicity to a chlorophycean alga, Chlorella. Ecotoxicol Environ Safe 59:223–227
Suh Y.-A., Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD (1999) Cell transformation by the superoxide-generating oxidase Mox1. Nature 401:79–82
Takahama U, Oniki T (1992) Regulation of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol 33:279–387
Tewari RK, Kumar P, Sharma PN (2006) Antioxidant responses to enhanced generation of superoxide anion radical and hydrogen peroxide in the copper-stressed mulberry plants. Planta 223:1145–1153
Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25:434–456
Wink DA, Cook JA, Pacelli R, Liebmann J, Krishne MC, Mitchell JB (1995) Nitric oxide (NO) protects against cellular damage by reactive oxygen species. Toxicol Lett 82–83:221–226
Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550
Yu KW, Gao WY, Son SH, Paek KY (2000) Improvement of ginsenoside production by jasmonic acid and some other elicitors in hairy root culture of ginseng (Panax ginseng C.A. Meyer). In Vitro Cell Develop Biol Plant 36:424–428
Yu CC, Hung KT, Kao CH (2005) Nitric oxide reduces Cu toxicity and Cu-induced NH +4 accumulation in rice leaves. J Plant Physiol 162:1319–1330
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Acknowledgments
This laboratory work is financially supported by the Ministry of Education and Human Resource Development (MOE), the Ministry of Commerce, Industry and Energy (MOCIE), Ministry of Labor (MOLAB) and the Korea Science and Engineering Foundation (KOSEF) grant funded by Korea government (MOST). Special thanks are given to Mrs. J-S Jeon for helpful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W.T. Kim.
Rights and permissions
About this article
Cite this article
Tewari, R.K., Hahn, EJ. & Paek, KY. Modulation of copper toxicity-induced oxidative damage by nitric oxide supply in the adventitious roots of Panax ginseng . Plant Cell Rep 27, 171–181 (2008). https://doi.org/10.1007/s00299-007-0423-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0423-7