Summary
Synopsis
Remoxipride is a substituted benzamide of the same class as sulpiride, and has a pharmacodynamic profile consistent with central antidopaminergic activity. It is a weak, but relatively selective, central dopamine D2-receptor antagonist and appears to have preferential affinity for extrastriatal dopamine D2-receptors. It also has marked affibrillationnity for central sigma receptors. Clinical data from noncomparative and comparative studies show that remoxipride has antipsychotic activity in patients with chronic schizophrenia, and acute exacerbation of chronic schizophrenia, with activity on both positive and negative symptoms. Its overall efficacy in these studies was similar to that of haloperidol. Importantly, however, remoxipride produced a substantially lower incidence of extrapyramidal effects than haloperidol. Further long term comparative studies are required to ascertain the relative suitability of remoxipride for preventing relapse in psychotic patients, and to determine whether tardive dyskinesia occurs in remoxipride recipients — the latter has not been reported with remoxipride to date
Thus, while further experience (particularly of a long term comparative nature) is needed, at present remoxipride appears to offer an important tolerability advantage over haloperidol
Pharmacodynamic Properties
Compared with the standard antipsychotics, remoxipride is a weak, but relatively specific central dopamine D2-receptor antagonist, with minimal effect on central cholinergic, serotonergic, histamine, muscarinic or α1-adrenergic receptors. Remoxipride appears to have little affibrillationnity for dopamine D1-receptors, as demonstrated by in vivo receptor binding studies and a lack of effect on dopamine-stimulated adenylate cyclase activity. However, remoxipride also has marked affinity for sigma receptors
While there is a need for caution with regard to extrapolation from in vitro studies to humans, observations that remoxipride induces differential increases in dopamine turnover in specific areas of rat brain indicate a preferential affinity for extrastriatal dopaminergic systems. This specificity for particular central dopaminergic systems is also demonstrated by the wide separation of doses of remoxipride effective in animal behavioural models for induction of catalepsy, which is believed to be mediated by striatal dopaminergic systems, and the antagonism of dopamine agonist-induced hyperactivity, thought to be mediated through the mesolimbic system. Remoxipride transiently increases plasma prolactin levels in humans; however, fewer remoxipride than haloperidol recipients had trough plasma prolactin levels outside normal limits during short term studies
Pharmacokinetic Properties
Remoxipride is almost completely absorbed following oral administration in healthy subjects: absorption rate is limited only by the dissolution rate of the preparation. Bio-availability is greater than 90%, and distribution is rapid, with peak plasma levels reached within 1 to 2 hours of oral administration. Approximately 80% of the absorbed dose is bound to plasma protein. There is a dose-proportional relationship for maximum and steady-state plasma concentrations of remoxipride. The half-life of remoxipride is between 4 and 7 hours. Most of an oral dose is excreted in the urine; 10 to 40% is excreted unchanged and the remainder as metabolites
Elimination may be impaired in elderly patients, patients with severe renal dysfunction or severe liver disorders, and in patients who are slow debrisoquine metabolisers. Decreases in urinary pH increase both the elimination rate and the percentage of remoxipride excreted unchanged in urine
Therapeutic Use
Short term noncomparative studies in patients with mainly chronic schizophrenia have shown that individualised doses of remoxipride provide an effective replacement for previous antipsychotic treatment. Over 75% of patients display moderate or marked symptomatic improvement at doses up to 600 mg/day. Both positive and negative symptoms appear to respond well to remoxipride. Improvements have been reported for the positive symptoms of thought disturbance, hostility/suspiciousness and hallucinations, and the negative symptoms of emotional withdrawal and motor retardation
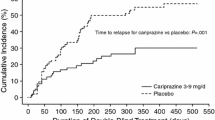
Most studies reported to date have compared the efficacy of remoxipride with haloperidol. In terms of overall therapeutic efficacy, remoxipride appears to be similar to both thioridazine and haloperidol but, importantly, it appears to produce a lower incidence of extrapyramidal symptoms. Data from a 6-month placebo-controlled trial show that doses of 150 to 300 mg/day were significantly superior to placebo in preventing relapse. However, the high withdrawal rate in the placebo group complicates interpretation of this study. In addition, data from 1 study comparing remoxipride with chlorpromazine and placebo suggest that remoxipride has a greater efficacy than placebo in patients responsive to treatment with other antipsychotics. Further studies with other antipsychotics are needed to extend these initial findings and confirm the place of remoxipride in the therapy of schizophrenia
Tolerability
Extrapyramidal symptoms are frequently associated with antipsychotic drug treatment and were present in the majority of patients before treatment with remoxipride. During treatment with remoxipride many of these patients showed a decrease in severity of such symptoms. There are relatively few reports of extrapyramidal effects attributable to remoxipride treatment. Few data are available on the effect of remoxipride on pre-existing tardive dyskinesia: preliminary results indicate either improvement or no effect on existing symptoms. The incidence of tardive dyskinesia with remoxipride, if any, has yet to be determined in longer term studies
Occasional cardiovascular effects, including postural hypotension, have been reported during remoxipride administration, but in most patients these would not be clinically important. Other miscellaneous reported adverse reactions occurred with a similar incidence in placebo and remoxipride recipients. Only a small number of patients were withdrawn from therapy due to adverse effects
Dosage and Administration
Oral administration of remoxipride should start with a dose of 300mg daily, in 2 divided doses, and the dosage should be adjusted to achieve maximum control of symptoms. The initial dosage should be halved for elderly patients. The majority of patients initially respond to doses of 300 to 450 mg/day although some require up to 600 mg/ day. For long term treatment, the lowest effective maintenance dose (usually 150 to 300 mg/day) should be administered
Similar content being viewed by others
References
Ahlfors UG, Rimõn R, Appelberg B, Hagert U, Harma P, et al. Remoxipride and haloperidol in schizophrenia: a double-blind multicentre study. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 99–103, 1990
Andersen J, Kørner A, Østergaard P, Fensbo C, Birket-Smith M, et al. A double blind comparative multicentre study of remoxipride and haloperidol in schizophrenia. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 104–107, 1990
Andersson U, Häggström J-E, Nilsson M-I, Widerlöv E. Remoxipride, a selective dopamine D2 receptor antagonist, in tardive dyskinesia. Psychopharmacology 94: 167–171, 1988
Andersson U, Nilsson M-I, Häggström J-E, Widerlöv E. Anti-dyskinetic action and pharmacokinetics of remoxipride, a substituted benzamide, in elderly patients with tardive dyskinesia. Presented at the 14th CINP Congress, Florence, 1984
Bergman J, Madras BK, Canfield D, Spealman RD. Behavioural suppressant effects of neuroleptics in monkeys: relation to dopamine D2 receptor binding. Abstract 053. Society for Neuroscience 13: 598, 1987
Chouinard G. Early phase II clinical trial of remoxipride in treatment of schizophrenia with measurements of prolactin and neuroleptic activity. Journal of Clinical Psychopharmacology 7: 159–164, 1987
Chouinard G. A placebo-controlled clinical trial of remoxipride and chlorpromazine in newly admitted schizophrenic patients with acute exacerbation. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 111–119, 1990
Classen W, Laux G. Comparison of sensorimotor and cognitive performance of acute schizophrenic inpatients treated with remoxipride or haloperidol. Neuropsychobiology 21: 131–140, 1989
Cooper SJ, King DJ, Blomqvist M, Doherty MM, Lindeberg A, et al. A 24 weeks’ relapse prevention study of remoxipride and placebo in chronic schizophrenic patients. Abstract. 17th Congress of Collegium Internationale Neuro-Psychopharmacologicum, Kyoto, Japan, September 10–14, 1990, Vol. 11, p. 249, 1990
den Boer JA, Ravelli DP, Huisman J, Öhrvik J, Verhoeven WMA, et al. A double-blind comparative study of remoxipride and haloperidol in acute schizophrenia. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 108–110, 1990
den Boer JA, Verhoeven WMA, Westenberg HGM. Remoxipride in schizophrenia. A preliminary report. Acta Psychiatrica Scandinavica 74: 409–414, 1986
Deo R, Soni S, Rastogi SC, Levine S, Plant I, et al. A double-blind comparative trial of remoxipride and haloperidol in the treatment of schizophrenia. Human Psychopharmacology 5: 133–141, 1990
Farde L, Grind M, Nilsson M-I, Ogenstad S, Sedvall G. Remoxipride — a new potential antipsychotic drug. Psychopharmacology 95: 157–161, 1988
Farde L, von Bahr C. Distribution of remoxipride to the human brain and central D2-dopamine receptor binding examined in vivo by PET. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 67–71, 1990
Farmer AE, McGuffin P. The pathogenesis and management of schizophrenia. Drugs 35: 177–185, 1988
Fog R. On stereotypy and catalepsy: studies on the effect of amphetamine and neuroleptics in rats. Acta Neurologica Scandinavica 48 (Suppl. 50): 1–66, 1972
Graffner C, Wagner Z, Nilsson M-I, Widerlöv E. Plasma concentrations of remoxipride and the gastrointestinal transit of 111In-marked extended-release coated spheres. Pharmaceutical Research 7: 54–58, 1990
Grind M, Nilsson M-I, Nilsson L, Oxenstierna G, Sedvall G, et al. Remoxipride — a new potential antipsychotic compound. Tolerability and pharmacokinetics after single oral and intravenous administration in healthy male volunteers. Psychopharmacology 98: 304–309, 1989
Groves PM, Rebec GV. Biochemistry and behavior: some central actions of amphetamine and antipsychotic drugs. Annual Review of Psychology 27: 91–127, 1976
Hall H, Sällemark M. Effects of chronic neuroleptic treatment on agonist affinity states of the dopamine-D2 receptor in the rat brain. Pharmacology and Toxicology 60: 359–363, 1987
Hall H, Sällemark M, Jerning E. Effects of remoxipride and some related new substituted salicylamides on rat brain receptors. Acta Pharmacologica et Toxicologica 58(1): 61–70, 1986
Högberg T, Ramsby S, de Paulis T, Stensland B, Csoregh I, et al. Solid state conformations and antidopaminergic effects of remoxipride hydrochloride and a closely related salicylamide. FLA 797, in relation to dopamine receptor models. Molecular Pharmacology 30: 345–351, 1986
Jostell K-G, Lapierre YD, the Canadian Remoxipride Study Group. Plasma concentration of remoxipride in relation to antipsychotic effect and adverse symptoms. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 48–50, 1990
Kahn J-P, Yisak W, Albaret C, Nilsson L, Zaar-Hedin A, et al. Tolerability and pharmacokinetics of remoxipride after intramuscular administration. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 51–53, 1990
Kelly PH, Iversen SD. Selective 60HDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulantinduced locomotor activity in rats. European Journal of Pharmacology 40: 45–56, 1976
Köhler C, Hall H, Magnusson O, Lewander T, Gustafsson K. Biochemical pharmacology of the atypical neuroleptic remoxipride. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 27–36, 1990
Lapierre YD, Nair NPV, Chouinard G, Awad AG, Saxena B, et al. A controlled dose-ranging study of remoxipride and haloperidol in schizophrenia — a Canadian multicentre trial. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 72–76, 1990
Laux G, Klieser E, Schröder HG, Dittmann V, Unterweger B, et al. A double-blind multicentre study comparing remoxipride, two and three times daily, with haloperidol in schizophrenia. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 125–129, 1990
Lewander T, Westerbergh S-E, Morrison D. Clinical profile of remoxipride — a combined analysis of a comparative double-blind multicentre trial programme. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 92–98, 1990
Lindström L, Besev G, Stening G, Widerlöv E. An open study of remoxipride, a benzamide derivative, in schizophrenia. Psychopharmacology 86: 241–243, 1985a
Lindström LH, Besev G, Stening G, Nilsson MI, Widerlöv E. The effect of remoxipride, a novel dopamine-D2 receptor blocker, in chronic schizophrenia. An open study. Presented at the IVth World Congress of Biological Psychiatry, 321.9, 1985b
Lindström LH, Wieselgren I-M, Struwe G, Kristiansson E, Akselson S, et al. A double-blind comparative multicentre study of remoxipride and haloperidol in schizophrenia. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 130–135, 1990
Losonczy MF, Davidson M, Davis KL. The dopamine hypothesis of schizophrenia. Psychopharmacology 70: 715–726, 1987
Lund Laursen A, Gerlach J. Remoxipride, a new substituted benzamide: an atypical antipsychotic drug with few side effects. Abstract 008. Presented at the 14th CINP Congress, Florence: 630, 1984
Lund Laursen A, Gerlach J. Antipsychotic effect of remoxipride, a new substituted benzamide with selective antidopaminergic activity. Acta Psychiatrica Scandinavica 73: 17–21, 1986
Magnusson O, Fowler CJ. Comparison of the effects of the novel antipsychotic agent remoxipride on dopamine and noradrenaline turnover in the rat brain. Pharmacology and Toxicology 65: 295–298, 1989
Magnusson O, Fowler CJ, Köhler C, ÖPgren S-O. Dopamine D2 receptors and dopamine metabolism. Relationship between biochemical and behavioural effects of substituted benzamide drugs. Neuropharmacology 25(2): 187–197, 1986
Magnusson O, Mohringe B, Thorell G, Lake-Bakaar DM. Effects of the dopamine D2 selective receptor antagonist remoxipride on dopamine turnover in the rat brain after acute and repeated administration. Pharmacology and Toxicology 60(5): 368–373, 1987
Mattila MJ, Mattila ME. Effects of remoxipride on psychomotor performance, alone and in combination with ethanol and diazepam. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 54–55, 1990
McCreadie RG, Morrison D, Eccleston D, Gall RG, Loudon J, et al. An open multicentre study of the treatment of florid schizophrenia with remoxipride. Acta Psychiatrica Scandinavica 72(2): 139–143, 1985
McCreadie RG, Todd N, Livingston M, Eccleston D, Watt JAG, et al. A double blind comparative study of remoxipride and thioridazine in the acute phase of schizophrenia. Acta Psychiatrica Scandinavica 78: 49–56, 1988
Mendlewicz J, de Bleeker E, Cosyns P, Deleu G, Lotstra F, et al. A double-blind comparative study of remoxipride and haloperidol in schizophrenic and schizophreniform disorders. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 138–141, 1990
Morrison D, Englund A, Lawrie V, Lewander T, Schlachet A, et al. Safety evaluation in both short-and long-term treatment of schizophrenia with remoxipride. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 164–169, 1990
Movin G, Gustafson L, Franzén G, Widerlöv E, Soni SD, et al. Pharmacokinetics of remoxipride in elderly psychotic patients. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 176–180, 1990
Nicklasson M, Graffner C, Nilsson L, Nilsson MI, Wahlen A. Absorption properties of the new potential antipsychotic drug remoxipride after oral administration to healthy volunteers. Pharmazeutische Industrie 9: 986–990, 1985
Nilsson MI, Wahlen A, Nilsson L, Vinnars E. Single dose pharmacokinetics of remoxipride — a new neuroleptic drug — in healthy volunteers. Clinical Pharmacology and Therapeutics 35(2): 263/B21, 1984
Ögren S-O, Florvall L, Hall H, Magnusson O, Ängeby-Möller K. Neuropharmacological and behavioural properties of remoxipride in the rat. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 21–26, 1990
Ögren SO, Hall H, Köhler C, Magnusson O. Neuropharmacological properties of remoxipride. Pharmacopsychiatry 21: 65, 1988
Ögren SO, Hall H, Köhler C, Magnusson O, Lindbom L-O, et al. Remoxipride, a new potential antipsychotic compound with selective antidopaminergic actions in the rat brain. European Journal of Pharmacology 102: 459–474, 1984
Patris M, Agussol P, Alby JM, Brion S, Burnat G, et al. A double-blind multicentre comparison of remoxipride, at two dose levels, and haloperidol. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 78–82, 1990
Pflug B, Bartels M, Bauer H, Bunse J, Gallhofer B, et al. A double-blind multicentre study comparing remoxipride, controlled release formulation, with haloperidol in schizophrenia. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 142–146, 1990
Phanjoo AL, Link C. Remoxipride versus thioridazine in elderly psychotic patients. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 181–185, 1990
Pijnenburg AJJ, Honig WMM, Van der Heyden JAM, Van Rossum JM. Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. European Journal of Pharmacology 35: 45–58, 1976
Rifkin A, Siris S. Drug treatment of acute schizophrenia. Psychopharmacology 112: 1095–1128, 1987
Segerberg-Konttinen M, Vuori E, Lukkari I, Penttilä A. Fatal intoxication by remoxipride. Journal of Forensic Sciences 34: 500–503, 1989
Snyder SH. Dopamine receptors, neuroleptics, and schizophrenia. American Journal of Psychiatry 138: 460–464, 1981
Snyder SH, Largent BL. Receptor mechanisms in antipsychotic drug action: focus on sigma receptors. Journal of Neuropsychiatry 1: 7–15, 1989
Stahle L, Ljungberg T, Rodebjer A, Ögren S-O, Ungerstedt U. Differential effects of the dopamine antagonist remoxipride on apomorphine induced behaviour in the rat. Pharmacology and Toxicology 60(3): 227–232, 1987
Strauss WH, Klieser E. Cognitive disturbances in neuroleptic therapy. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 56–57, 1990
Tamminga CA, Gerlach J. New neuroleptics and experimental antipsychotics in schizophrenia. Psychopharmacology 116: 1129–1140, 1987
Tench D, Soni SD, Ashwood T, Movin G. Steady-state pharmacokinetics of controlled release and intermediate release formulations of remoxipride in patients with chronic schizophrenia. Psychopharmacology 101: 132–136, 1990
von Bahr C, Movin G, Yisak W-A, Jostell K-G, Widman M. Clinical pharmacokinetics of remoxipride. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 41–44, 1990
Wâlinder J, Holm A-C. Experiences of long-term treatment with remoxipride: efficacy and tolerability. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 158–163, 1990
Widerlöv E, Franzén G, Jansson P, Movin G. Pharmacokinetics of remoxipride controlled release and immediate release capsules in schizophrenic patients. International Clinical Psychopharmacology 5: 125–134, 1990
Widerlöv E, Termander B, Nilsson M-I. Effect of urinary pH on the plasma and urinary kinetics of remoxipride in man. European Journal of Clinical Pharmacology 37: 359–363, 1989
Widman M, Bryske B, Movin G, Nilsson L, Nilsson M-I. Pharmacokinetics of remoxipride and metabolites following a single oral dose of 14C-remoxipride. Abstract 245. XIth International Congress of Pharmacology, Amsterdam, July 1–6, 1990
Yisak W, von Bahr C, Farde L, Gram LE, Grind M, et al. Remoxipride: drug interaction studies. Abstract. Association of European Psychiatrists Fifth European Congress, Strasbourg, 17–20 October, 1990b
Yisak W, von Bahr C, Farde L, Grind M, Mattila M, et al. Drug interaction studies with remoxipride. Acta Psychiatrica Scandinavica 82 (Suppl. 358): 58–62, 1990a
Zemlan FP, Hitzemann RJ, Hirschowitz J, Garver DL. Down-regulation of central dopamine receptors in schizophrenia. American Journal of Psychiatry 142: 1334–1337, 1985
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: R.J. Baldessarini, Mailman Research Center, McLean Hospital, Belmont, Massachusetts, USA; G. Chouinard, Allan Memorial Institute, Montreal, Quebec, Canada; S. Gershon, University of Pittsburgh, Western Psychiatric Institute and Clinic, Pittsburgh, Pennsylvania, USA; L.H. Hollister, Harris County Psychiatric Center, Houston, Texas, USA; D.J. King, Department of Therapeutics and Pharmacology, The Queen’s University of Belfast, Belfast, Northern Ireland; M.H. Lader, Institute of Psychiatry, University of London, London, England; L. Lindström, University of Uppsala Psychiatric Research Center, Uppsala, Sweden; R.G. McCreadie, Department of Clinical Research, Crichton Royal Hospital, Dumfries, Scotland; S.H. Snyder, Department of Neuroscience, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; M. Tanaka, Department of Pharmacology, Kurume University School of Medicine, Kurume, Japan; T. Yanagita, 1st Department of Pharmacology, The Jikei University School of Medicine, Kawasaki, Japan
Rights and permissions
About this article
Cite this article
Wadworth, A.N., Heel, R.C. Remoxipride. Drugs 40, 863–879 (1990). https://doi.org/10.2165/00003495-199040060-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199040060-00008