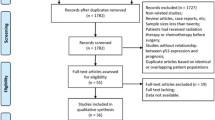
Abstract
Background
The value of p53 status for predicting response to chemotherapy-based treatment in patients with esophageal cancer has been controversial. We conducted a meta-analysis to elucidate the correlation of p53 status with the response to chemotherapy-based treatment.
Methods
Studies were searched in PubMed, Embase, and Web of Science (up to September 2012). The p53 status and response to therapy were defined and standardized. Subgroup analyses based on the treatment and histopathology were performed to explore the usefulness of p53 status for predicting response to therapy in esophageal cancer. Sensitivity analyses were conducted by removing specific studies to assess the effects of study quality.
Results
We included 28 studies with 1497 cases in our meta-analysis. Wild-type form of p53 status (low expression of p53 protein and/or wild-type p53 gene) was associated with high response to chemotherapy-based treatment in esophageal cancer (total major response [MR]: risk ratio [RR] = 1.09, 95 % CI = 1.03–1.16, P = .003; pathological MR: RR = 1.15, 95 % CI = 1.06–1.25, P = .001; total complete response [CR]: RR = 1.08, 95 % CI = 1.00–1.17, P = .040). The similar correlation between the wild-type form p53 and response to therapy were also detected in subgroup analyses (total MR, pathological MR, and total CR in chemoradiotherapy subgroup; total MR in chemotherapy subgroup; total MR and pathological CR in esophageal squamous cell carcinoma [ESCC]). Additionally, patients with wild-type form p53 status had high pathological complete response rate to neoadjuvant chemoradiotherapy in ESCC.
Conclusions
The current meta-analysis suggested that p53 status might be a predictive biomarker for response to chemotherapy-based treatment in esophageal cancer.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52.
Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet Oncol. 2002;359:1727–33.
Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–7.
Brucher BL, Stein HJ, Zimmerman F, Werner M, Sarbia M, Busch R, et al. Responders benefit from neoadjuvant radiochemotherapy in esophageal squamous cell carcinoma: results of a prospective phase-II trial. Eur J Surg Oncol. 2004;30:963–71.
Fareed KR, Kaye P, Soomro IN, Ilyas M, Martin S, Parsons SL, et al. Biomarkers of response to therapy in oesophago-gastric cancer. Gut. 2009;58:127–43.
Makino T, Yamasaki M, Miyata H, Yoshioka S, Takiguchi S, Fujiwara Y, et al. p53 mutation status predicts pathological response to chemoradiotherapy in locally advanced esophageal cancer. Ann Surg Oncol. 2010;17:804–11.
Lam KY. Tsao SW, Zahng D, Law S, He D, Ma L, et al. Prevalence and predictive value of p53 mutation in patients with esophageal squamous cell carcinomas: prospective clinico-pathological study and survival analysis of 70 patients. Int J Cancer. 1997;74:212–9.
Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;37:413–31.
Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, et al. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807–10.
Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–67.
Levine AJ. p53, cellular gatekeeper for growth and division. Cell. 1997;88:323–31.
Weller M. Predicting response to cancer chemotherapy: the role of p53. Cell Tissue Res. 1998;292:435–45.
Makino T, Yamasaki M, Miyata H, Yoshioka S, Takiguchi S, Fujiwara Y, et al. p53 mutation status predicts pathological response to chemoradiotherapy in locally advanced esophageal cancer. Ann Surg Oncol. 2009;17:804–11.
Yamasaki M, Miyata H, Fujiwara Y, Takiguchi S, Nakajima K, Nishida T, et al. p53 genotype predicts response to chemotherapy in patients with squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2010;17:634–42.
Okumura H, Natsugoe S, Matsumoto M, Mataki Y, Takatori H, Ishigami S, et al. The predictive value of p53, p53R2, and p21 for the effect of chemoradiation therapy on oesophageal squamous cell carcinoma. Br J Cancer. 2005;92:284–9.
Yang B, Rice TW, Adelstein DJ, Rybicki LA, Goldblum JR. Overexpression of p53 protein associates decreased response to chemoradiotherapy in patients with esophageal carcinoma. Mod Pathol. 1999;12:251–6.
Sunada F, Itabashi M, Ohkura H, Okumura T. p53 negativity, CDC25B positivity, and metallothionein negativity are predictors of a response of esophageal squamous cell carcinoma to chemoradiotherapy. World J Gastroenterol. 2005;11:5696–700.
Yamamoto Y, Yamai H, Seike J, Yoshida T, Takechi H, Furukita Y, et al. Prognosis of esophageal squamous cell carcinoma in patients positive for human epidermal growth factor receptor family can be improved by initial chemotherapy with docetaxel, fluorouracil, and cisplatin. Ann Surg Oncol. 2012;19:757–65.
Kishi K, Doki Y, Yano M, Yasuda T, Fujiwara Y, Takiguchi S, et al. Reduced MLH1 expression after chemotherapy is an indicator for poor prognosis in esophageal cancers. Clin Cancer Res. 2003;9:4368–75.
Fukuchi M, Fukai Y, Sohda M, Miyazaki T, Nakajima M, Inose T, et al. Expression of the prolyl isomerase Pin1 is a useful indicator of sensitivity to chemoradiotherapy in advanced esophageal squamous cell carcinoma. Oncology Rep. 2009;21:853–9.
Nam TK, Lee JH, Cho SH, Chung IJ, Ahn SJ, Song JY, et al. Low hMLH1 expression prior to definitive chemoradiotherapy predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Lett. 2008;260:109–17.
Sarbia M, Ott N, Pühringer-Oppermann F, Brücher BLDM. The predictive value of molecular markers (p53, EGFR, ATM, CHK2) in multimodally treated squamous cell carcinoma of the oesophagus. Br J Cancer. 2007;97:1404–8.
Sohda M, Ishikawa H, Masuda N, Kato H, Miyazaki T, Nakajima M, et al. Pretreatment evaluation of combined HIF-1alpha, p53 and p21 expression is a useful and sensitive indicator of response to radiation and chemotherapy in esophageal cancer. Int J Cancer. 2004;110:838–44.
Pakos EE, Kyzas PA, Ioannidis JP. Prognostic significance of TP53 tumor suppressor gene expression and mutations in human osteosarcoma: a meta-analysis. Clin Cancer Res. 2004;10:6208–14.
Hall PA, Lane DP. P53 in tumour pathology: can we trust immunohistochemistry? J Pathol. 1994;172:1–4.
Kandioler-Eckersberger D, Ludwig C, Rudas M, Kappel S, Janschek E, Wenzel C, et al. TP53 mutation and p53 overexpression for prediction of response to neoadjuvant treatment in breast cancer patients. Clin Cancer Res. 2000;6:50–6.
Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–40.
Japanese Society for Esophageal Diseases. Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus. 9th ed. Tokyo: Kanehara & Co, Ltd; 1999.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Puglisi F, Di Loreto C, Panizzo R, Avellini C, Fongione S, Cacitti V, et al. Expression of p53 and bcl-2 and response to preoperative chemotherapy and radiotherapy for ESCC. J Clin Pathol. 1996;49:456–9.
Muro K, Ohtsu A, Boku N, Chin K, Oda Y, Fujii T, et al. Association of p53 protein expression with responses and survival of patients with locally advanced esophageal carcinoma treated with chemoradiotherapy. Jpn J Clin Oncol. 1996;26:65–9.
Krasna MJ, Mao YS, Sonett JR, Tamura G, Jones R, Suntharalingam M, et al. P53 gene protein overexpression predicts results of trimodality therapy in esophageal cancer patients. Ann Thorac Surg. 1999;68:2021–5.
Samejima R, Kitajima Y, Yunotani S, Miyazaki K. Cyclin D1 is a possible predictor of sensitivity to chemoradiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 1999;19:5515–21.
Shimada Y, Watanabe G, Yamasaki S, Maeda M, Kawabe A, Kaganoi JI, et al. Histological response of cisplatin predicts patients’ survival in oesophageal cancer and p53 protein accumulation. Eur J Cancer. 2000;36:987–93.
Szumilo J, Chibowski D, D browski A. Assessment of the predictive value of clinical and histopathological factors as well as the immunoexpression of p53 and bcl-2 proteins in response to preoperative chemotherapy. Dis Esophagus. 2000;13:191–7.
Takeno S, Noguchi T, Takahashi, Kikuchi R, Uchida Y, Yokoyama S. Immunohistochemical and clinicopathologic analysis of response to neoadjuvant therapy for esophageal squamous cell carcinoma. Dis Esophagus. 2001;14:149–54.
Ito T, Kaneko K, Makino R, Ito H, Konishi K, Kurahashi T, et al. Prognostic value of p53 mutations in patients with locally advanced esophageal carcinoma treated with definitive chemoradiotherapy. J Gastroenterol. 2001;36:303–11.
Kajiyama Y, Hattori K, Tomita N, Amano T, Iwanuma Y, Narumi K, et al. Histopathologic effects of neoadjuvant therapies for advanced squamous cell carcinoma of the esophagus multivariate analysis of predictive factors and p53 overexpression. Dis Esophagus. 2002;15:61–6.
Shimada H, Hoshino T, Okazumi S, Matsubara H, Funami Y, Nabeya Y, et al. Expression of angiogenic factors predicts response to chemoradiotherapy and prognosis of oesophageal squamous cell carcinoma. Br J Cancer. 2002;86:552–7.
Michel P, Magois K, Robert V, Chiron A, Lepessot F, Bodenant C, et al. Prognostic value of TP53 transcriptional activity on p21 and bax in patients with esophageal squamous cell carcinomas treated by definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:379–85.
Beardsmore DM, Verbeke CS, Davies CL, Guillou PJ, Clark GW. Apoptotic and proliferative indexes in esophageal cancer predictors of response to neoadjuvant therapy. J Gastrointest Surg. 2003;7:77–87.
Takeuchi H, Ozawa S, Ando N, Kitagawa Y, Ueda M, Kitajima M. Cell-cycle regulators and the Ki-67 labeling index can predict the response to chemoradiotherapy and the survival of patients with locally advanced squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2003;10:792–800.
Heeren PA, Kloppenberg FW, Hollema H, Mulder NH, Nap RE, Plukker JT. Predictive effect of p53 and p21 alteration on chemotherapy response and survival in locally advanced adenocarcinoma of the esophagus. Anticancer Res. 2004;24:2579–83.
Nakamura T, Hayashi K, Ota, M, Ide H, Takasaki K, Mitsuhashi M. Expression of p21 predicts response and survival of esophageal cancer patients treated by chemoradiotherapy. Dis Esophagus. 2004;17:315–21.
Miyazaki T, Kato H, Faried A, Sohda M, Nakajima M, Fukai Y, et al. Predictors of response to chemo-radiotherapy and radiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2005;25:2749–55.
Ishida M, Morita M, Saeki H, Ohga T, Sadanaga N, Watanabe M, et al. Expression of p53 and p21 and the clinical response for hyperthermochemoradiotherapy in patients with squamous cell carcinoma of the esophagus. Anticancer Res. 2007;27:3501–6.
Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9.
Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma. Cancer. 2001;91:2165–74.
Nasierowska-Guttmejer A, Szawlowski AW, Falkowski S, Morysinski T. Histopathological evaluation of response to pre-operative concurrent chemoradiotherapy for advanced squamous cell carcinoma of the thoracic esophagus. Dis Esophagus. 1995;8:136–41.
Acknowledgements
This work was supported by of Chinese Ministry of Health Key Program grant (No. 179). We would like to thank the authors of the studies included in our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Shui-Shen Zhang and Qing-Yuan Huang contributed equally to this work and share first authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2012_2859_MOESM2_ESM.tif
Supplement Figure 1: Forest plots of RR were estimated for association between p53 status and pathological complete response (CR) to neoadjuvant chemoradiotherapy (NCRT) in patients with esophageal squamous cell carcinoma. Supplementary material 2 (TIFF 53 kb)
10434_2012_2859_MOESM3_ESM.tif
Supplement Figure 2: The funnel plot shows that there was no obvious indication of publication bias for the outcome of total major response (MR) to chemotherapy-based treatment in patients with esophageal cancer. Supplementary material 3 (TIFF 66 kb)
Rights and permissions
About this article
Cite this article
Zhang, SS., Huang, QY., Yang, H. et al. Correlation of p53 Status with the Response to Chemotherapy-Based Treatment in Esophageal Cancer: A Meta-Analysis. Ann Surg Oncol 20, 2419–2427 (2013). https://doi.org/10.1245/s10434-012-2859-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-012-2859-4