Abstract
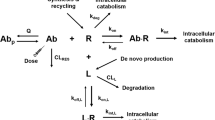
Interleukin-12 (IL12) is a cytokine with potential applications in the treatment of cancer given the potent immune response that it triggers, in part due to its ability to stimulate expression of interferon-γ (IFNγ). To avoid the toxicity associated with systemic exposure to IL12, a high-capacity adenoviral vector carrying a liver-specific, mifepristone-inducible IL12 expression system (HC-Ad/RUmIL12) has been developed. However, the maintenance of IL12 expression at therapeutic levels is compromised by the inhibitory effect of IFNγ on inducible systems. The aim of this work is to develop a semi-mechanistic model to characterize the relationship between IL12 and IFNγ in wild-type and knock-out mice for the IFNγ receptor treated with HC-Ad/RUmIL12 under different dosing regimens in order to better understand the key mechanisms controlling the system. Rapid binding was considered to account for target-mediated disposition exhibited by both cytokines (equilibrium dissociation constant were 18 and 2.28 pM for IL12 and IFNγ, respectively). The final model included: (1) IFNγ receptor turnover, (2) irreversible free cytokine elimination from the serum compartment, (3) internalization of the IL12 receptor complex, (4) IL12 expression upregulated by the co-administration of the adenoviral vector and mifepristone and downregulated by the IFNγ receptor, and (5) synthesis of IFNγ controlled by the relative increments in the bound IL12. In conclusion, a model simultaneously describing the kinetics of IL12 and IFNγ in the context of gene therapy was developed and validated with additional data. The model was applied to design an experimental dosing protocol intended to maintain sustained therapeutic IL12 levels.







Similar content being viewed by others
Notes
http://getdata-graph-digitizer.com/
Abbreviations
- −2LL:
-
−2 × log (likelihood)
- E MAX :
-
Maximum effect
- IFNγ:
-
Interferon-γ
- IL12:
-
Interleukin-12
- HC-Ad/RUmIL12:
-
Adenoviral vector carrying a liver-specific, mifepristone-inducible IL12 expression system
- KO:
-
Knock-out
- mIL12:
-
Mouse interleukin-12
- NK:
-
Natural killers
- REG:
-
Regulator compartment
- RU:
-
Mifepristone
- RU50 :
-
Mifepristone concentration needed to achieve 50% of the maximum effect
- TMDD:
-
Target-mediated drug disposition
- WT:
-
Wild-type
References
Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13(1):251–76.
Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146(9):3074–84.
Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–68.
Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13(16):4677–85.
Sangro B, Melero I, Qian C, Prieto J. Gene therapy of cancer based on interleukin 12. Curr Gene Ther. 2005;5(6):573–81.
Berraondo P, Prieto J, Gonzalez-Aseguinolaza G. Advances in interleukin-12 gene therapy for acquired liver diseases. Curr Gene Ther. 2009;9(2):62–71.
Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ, Bromberg JS. Promoter attenuation in gene therapy: interferon-γ and tumor necrosis factor-α inhibit transgene expression. Hum Gene Ther. 1997;8(17):2019–29.
Wang L, Hernández-Alcoceba R, Shankar V, Zabala M, Kochanek S, Sangro B, et al. Prolonged and inducible transgene expression in the liver using gutless adenovirus: a potential therapy for liver cancer. Gastroenterology. 2004;126(1):278–89.
Burcin MM, Schiedner G, Kochanek S, Tsai SY, O’Malley BW. Adenovirus-mediated regulable target gene expression in vivo. Proc Natl Acad Sci USA. 1999;96(2):355–60.
Gonzalez-Aparicio M, Alzuguren P, Mauleon I, Medina-Echeverz J, Hervas-Stubbs S, Mancheno U, et al. Oxaliplatin in combination with liver-specific expression of interleukin 12 reduces the immunosuppressive microenvironment of tumours and eradicates metastatic colorectal cancer in mice. Gut. 2011;60(3):341–9.
Reboredo M, Zabala M, Mauleon I, De LR, Kreppel F, Kochanek S, et al. Interleukin-12 inhibits liver-specific drug-inducible systems in vivo. Gene Ther. 2007;15(4):277–88.
Zhang L, Pfister M, Meibohm B. Concepts and challenges in quantitative pharmacology and model-based drug development. APPS J. 2008;10(4):552–9.
Agur Z, Vuk-Pavlovic S. Mathematical modeling in immunotherapy of cancer: personalizing clinical trials. Mol Ther. 2012;20(1):1–2.
Crettaz J, Berraondo P, Mauleón I, Ochoa L, Shankar V, Barajas M, et al. Intrahepatic injection of adenovirus reduces inflammation and increases gene transfer and therapeutic effect in mice. Hepatology. 2006;44(3):623–32.
Beal S, Sheiner L, Boeckmann A. NONMEM Users Guide. Icon Development Solutions 1989–2006.
Babij P, Psaltis G, Song D, Kulik J, Mollova N, Abruzzese RV, et al. “Blue heart”: characterization of a mifepristone-dependent system for conditional gene expression in genetically modified animals. Biochim Biophys Acta. 2003;1627(1):15–25.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–32.
Mager D, Krzyzanski W. Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res. 2005;22(10):1589–96.
Lindbom L, Pihlgren P, Jonsson N. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241–57.
Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Pharmacodyn. 1994;22(5):431–45.
Wu C, Warrier RR, Carvajal DM, Chua AO, Minetti LJ, Chizzonite R, et al. Biological function and distribution of human interleukin-12 receptor ß chain. Eur J Immunol. 1996;26(2):345–50.
Wu C, Wang X, Gadina M, O’Shea JJ, Presky DH, Magram J. IL-12 receptor β2 (IL-12Rβ2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J Immunol. 2000;165(11):6221–8.
Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56(3):248–52.
Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn. 2008;35(5):573–91.
Meibohm B. Population pharmacokinetic/pharmacodynamic analyses as the basis for dosing of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2011;50(12):823–4.
Rakhit A, Yeon MM, Ferrante J, Fettner S, Nadeau R, Motzer R, et al. Down-regulation of the pharmacokinetic-pharmacodynamic response to interleukin-12 during long-term administration to patients with renal cell carcinoma and evaluation of the mechanism of this “adaptive response” in mice. Clin Pharmacol Ther. 1999;65(6):615–29.
Li R, Deng F, Fu Y, Zhang Y, Wan H, Zhou D, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection and intravenous infusion of recombinant human interleukin-12 and recombinant human interleukin-12 combined with hepatitis B surface antigen in cynomolgus monkeys. Pharmacology. 2010;85(6):319–27.
Zeuzem S, Hopf U, Carreno V, Diago M, Shiffman M, Grüne S, et al. A phase I/II study of recombinant human interleukin-12 in patients with chronic hepatitis C. Hepatology. 1999;29(4):1280–7.
Abraham AK, Kagan L, Kumar S, Mager DE. Type I interferon receptor is a primary regulator of target-mediated drug disposition of interferon-β in mice. J Pharmacol Exp Ther. 2010;334(1):327–32.
Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi JH, et al. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther. 2002;72(1):20–32.
Nadeau RR, Ostrowski C, Ni-Wu G, Liberato DJ. Pharmacokinetics and pharmacodynamics of recombinant human interleukin-12 in male rhesus monkeys. J Pharmacol Exp Ther. 1995;274(1):78–83.
Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995;146(7–8):423–31.
Finley SD, Gupta D, Cheng N, Klinke DJ. Inferring relevant control mechanisms for interleukin-12 signaling in naive CD4+ T cells. Immunol Cell Biol. 2011;89(1):100–10.
O’Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK. Role of IL-12 in the induction and potentiation of IFN-γ in response to bacillus calmette–guérin. J Immunol. 1999;163(8):4246–52.
Becskei A, Grusby MJ. Contribution of IL-12R mediated feedback loop to Th1 cell differentiation. FEBS Lett. 2007;581(27):5199–206.
Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202(1):8–32.
Ikeda H, Old LJ, Schreiber RD. The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13(2):95–109.
Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259(5102):1742–5.
Abraham AK, Krzyzanski W, Mager DE. Partial derivative—based sensitivity analysis of models describing target-mediated drug disposition. APPS J. 2007;9(2):181–9.
Bader T, Weitzerbin J. Nuclear accumulation of interferon gamma. Proc Natl Acad Sci USA. 1994;91(25):11831–5.
Cofano F, Moore SK, Tanaka S, Yuhki N, Landolfo S, Appella E. Affinity purification, peptide analysis, and cDNA sequence of the mouse interferon gamma receptor. J Biol Chem. 1990;265(7):4064–71.
Schreiber RD, Farrar MA, Hershey GK, Fernandez-Luna J. The structure and function of interferon-[gamma] receptors. Int J Immunopharmacol. 1992;14(3):413–9.
Ma P. Theoretical considerations of target-mediated drug disposition models: simplifications and approximations. Pharm Res. 2012;29(3):866–82.
Garg A, Balthasar JP. Investigation of the influence of FcRn on the distribution of IgG to the brain. AAPS J. 2009;11(3):553–7.
Acknowledgments
This work was supported by: (1) grant SAF2009-11324 from the Spanish Department of Science and the UTE project CIMA and the agreement FIMA, the “UTE project CIMA”, Fondo de Investigacion Sanitaria (FIS PI10/00264) and (2) from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115156, resources of which are composed of financial contributions from the European Union’s Seventh Framework Programme (FP7/2007–2013) and EFPIA companies’ in kind contribution. The DDMoRe project is also supported by financial contribution from Academic and SME partners. This work does not necessarily represent the view of all DDMoRe partners. Additionally, ZPP-G was supported by FPU fellowship from the Spanish Ministerio de Educacion, Cultura y Deporte. PB was supported by a Miguel Servet contract from Spanish Fondo de Investigacion Sanitaria
Author information
Authors and Affiliations
Corresponding author
ELECTRONIC SUPPLEMENTARY MATERIALs
Below is the link to the electronic supplementary material.
Supplemental Materials
Pharmacokinetic model developed for mifepristone and computational model developed for wild-type mice (DOC 78 kb)
Supplementary Fig. 1
Wild-type mice models. a Schematic representation of the Push-and-pull model, b together with the corresponding IL12 observed (Obs) and model predicted concentrations (Pred) vs time profiles obtained for the low and high viral dose in experiment 1. c Schematic representation of the TMDD model developed for wild-type mice along with the d IL12 observed and model predicted concentrations vs time profiles for the low and high viral dose corresponding to experiment 1 (upper panel) and the IL12 observed and predicted levels measured 10 h after the first administration of mifepristone vs the RU dose (lower panel) from experiments 1–4. Terms and parameters are described in the text (JPEG 75 kb)
Rights and permissions
About this article
Cite this article
Parra-Guillen, Z.P., Janda, A., Alzuguren, P. et al. Target-Mediated Disposition Model Describing the Dynamics of IL12 and IFNγ after Administration of a Mifepristone-Inducible Adenoviral Vector for IL-12 Expression in Mice. AAPS J 15, 183–194 (2013). https://doi.org/10.1208/s12248-012-9423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9423-9