Abstract
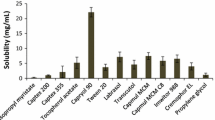
The main purpose of this work was to develop an oral microemulsion formulation for enhancing the bioavailability of acyclovir. A Labrafac-based microemulsion formulation with Labrasol as surfactant and Plurol Oleique as cosurfactant was developed for oral delivery of acyclovir. Phase behavior and solubilization capacity of the microemulsion system were characterized, and in vivo oral absorption of acyclovir from the microemulsion was investigated in rats. A single isotropic region, which was considered to be a bicontinuous microemulsion, was found in the pseudoternary phase diagrams developed at various Labrasol:Plurol Oleique:Labrafac ratios. With the increase of Labrasol concentration, the microemulsion region area and the amount of water and Labrafac solubilized into the microemulsion system increased; however, the increase of Plurol Oleique percentage produced opposite effects. The microemulsion system was also investigated in terms of other characteristics, such as interfacial tension, viscosity, pH, refractive index, diffusion, and bioavailability. Acyclovir, a poorly soluble drug, displayed high solubility in a microemulsion formulation using Labrafac (10%). Labrasol (32%), Plurol Oleique (8%), and water (50%). The in vitro intraduodenal diffusion and in vivo study revealed an increase of bioavailability (12.78 times) after oral administration of the microemulsion formulation as compared with the commercially available tablets.
Similar content being viewed by others
References
O’Brien J, Campoli-Richards D. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy.Drugs 1989;37:233–309.
McEvoy G.AHFS Drugs Information, American Society of Health System Pharmacists. Bethesda, MD: Atlantic Books; 1998:471–480.
Fletcher C, Bean B. Evaluation of oral acyclovir therapy.Drug Intell Clin Pharm. 1985;19:518–524.
Vergin H, Kikuta C, Mascher H, Metz R. Pharmacokinetics and bioavailability of different formulations of aciclovir.Arzneimittelforschung. 1995;45:508–515.
Charman S, Charman W, Rogge M, Wilson T, Dutko F, Pouton C. Self-emulsifying drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compounds.Pharm Res. 1992;9:87–93.
Constantinides P, Scalart J, Lancaster C, et al. Formulation and intestinal absorption enhancement evaluation of water-in-oil microemulsions incorporating medium-chain glycerides.Pharm Res. 1994;11:1385–1390.
Kovarik J, Muelle E, Van Bree J, Tetzloff W, Kutz K. Reduced inter- and intraindividual variability in cyclosporine pharmacokinetics from a microemulsion formulation.J Pharm Sci. 1994;83:444–446.
Shah N, Carvajal M, Patel C, Infeld M, Malick A. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs.Int J Pharm. 1994;106:15–23.
Matuszewska B, Hettrick L, Bondi J, Storey D. Comparative bioavailability of L-683,443, a 5a-reductase inhibitor, from a self-emulsifying drug delivery system in beagle dogs.Int J Pharm. 1996;136:147–154.
Garti N, Aserin A, Tiunova I, Fanun MA. DSC study of water behavior in water-in-oil microemulsions stabilized by sucrose esters and butanol.Colloids Surf B: Physicochem Eng Aspects. 2000;170:1–18.
Attwood D. Microemulsions. In: Kreuter J, ed.Colloidal Drug Delivery Systems. New York, NY: Marcel Dekker; 1994: 31–71.
Smith P. Methods for evaluating intestinal permeability and metabolism in vitro.Pharm Biotechnol. 1996;8:13–34.
Gibaldi M, Perrier D.Pharmacokinetics. New York, NY: Marcel-Dekker; 1982.
Ghosh PK, Umrethia M, Majithiya RJ, Murthy RSR. Preparation and physicochemical characterisation of caprylocapryl macrogol-8-glycerides microemulsion for oral drug delivery.Ars Pharm. 2004; 45:353–372.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: September 15, 2006
Rights and permissions
About this article
Cite this article
Ghosh, P.K., Majithiya, R.J., Umrethia, M.L. et al. Design and development of microemulsion drug delivery system of acyclovir for improvement of oral bioavailability. AAPS PharmSciTech 7, 77 (2006). https://doi.org/10.1208/pt070377
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/pt070377