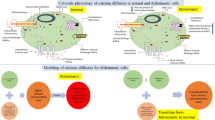
Abstract
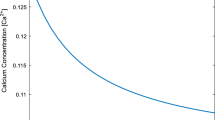
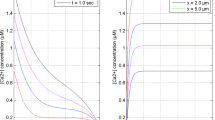
Calcium (Ca2+) signaling is essential for regulating dopamine (DA) concentration levels in neurons. The alterations in the different mechanisms of [Ca2+] dynamics can cause the disturbances in the dopamine regulation in neuron cells. But, no attempt is reported on the spatiotemporal dependence of dopamine regulation on the [Ca2+] dynamics in neurons. A mathematical model is framed to explore the effects of alterations in different processes including source influx, ryanodine receptor, buffer process, serca pump, etc. on the spatiotemporal [Ca2+] and dopamine mechanisms in nerve cells. The Numerical findings have been acquired utilizing finite element techniques and the effects of dysregulation in diverse processes on the spatiotemporal [Ca2+] and dopamine mechanisms in dopaminergic neurons are analyzed. The outcomes provide the better understanding of the regulatory and dysregulatory processes, which can cause the alterations in the spatiotemporal [Ca2+] and dopamine dynamics leading to neuronal diseases such as Parkinson’s, attention deficit hyperactive disorder, depression, schizophrenia, etc. Thus, the present model gives novel information about the specific dysregulatory constitutive mechanisms of calcium and dopamine dynamics like source inflow, buffer, ryanodine receptor, and others, which are responsible for the elevated calcium and dopamine levels, consequently the death of dopaminergic neurons.


















Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
H. Yuan, J.C. Zheng, P. Liu, S.F. Zhang, J.Y. Xu, L.M. Bai, Pathogenesis of Parkinson’s disease: Oxidative stress, environmental impact factors and inflammatory processes. Neurosci. Bull. 23, 125–130 (2007). https://doi.org/10.1007/s12264-007-0018-x
A.H. Stokes, T.G. Hastings, K.E. Vrana, Cytotoxic and genotoxic potential of dopamine. J. Neurosci. Res. 55, 659–665 (1999)
P. Wellstead, M. Cloutier, Systems biology of parkinson’s disease. Syst. Biol. Park. Dis. (2012). https://doi.org/10.1007/978-1-4614-3411-5
A. Atri, J. Amundson, D. Clapham, J. Sneyd, A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys. J. 65, 1727–1739 (1993). https://doi.org/10.1016/S0006-3495(93)81191-3
R. Llinas, The role of calcium in neuronal function. Neurosci. Fourth Study Progr. 2, 555–571 (1979)
A.L. Fogelson, R.S. Zucker, Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys. J. 48, 1003–1017 (1985). https://doi.org/10.1016/S0006-3495(85)83863-7
J. Wagner, J. Keizer, Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys. J. 67, 447–456 (1994). https://doi.org/10.1016/S0006-3495(94)80500-4
G.D. Smith, J. Wagner, J. Keizer, Validity of the rapid buffering approximation near a point source of calcium ions. Biophys. J. 70, 2527–2539 (1996). https://doi.org/10.1016/S0006-3495(96)79824-7
G.D. Smith, Analytical steady-state solution to the rapid buffering approximation near an open Ca2+ channel. Biophys. J. 71, 3064–3072 (1996). https://doi.org/10.1016/S0006-3495(96)79500-0
A. Verkhratsky, The endoplasmic reticulum and neuronal calcium signalling. Cell Calcium 32, 393–404 (2002). https://doi.org/10.1016/S0143416002001896
M. Falcke, Buffers and oscillations in intracellular Ca2+ dynamics. Biophys. J. 84, 28–41 (2003). https://doi.org/10.1016/S0006-3495(03)74830-9
J. Sneyd, K. Tsaneva-Atanasova, J.I.E. Bruce, S.V. Straub, D.R. Giovannucci, D.I. Yule, A model of calcium waves in pancreatic and parotid acinar cells. Biophys. J. 85, 1392–1405 (2003). https://doi.org/10.1016/S0006-3495(03)74572-X
M. Brini, T. Calì, D. Ottolini, E. Carafoli, Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 71, 2787–2814 (2014). https://doi.org/10.1007/s00018-013-1550-7
K.B. Pathak, N. Adlakha, Finite element model to study calcium signalling in cardiac myocytes involving pump, leak and excess buffer. J. Med. Imaging Heal. Inform. 5, 683–688 (2015). https://doi.org/10.1166/jmihi.2015.1443
K. Pathak, N. Adlakha, Finite element model to study two dimensional unsteady state calcium distribution in cardiac myocytes, Alexandria. J. Med. 52, 261–268 (2016). https://doi.org/10.1016/j.ajme.2015.09.007
N. Singh, N. Adlakha, A mathematical model for interdependent calcium and inositol 1,4,5-trisphosphate in cardiac myocyte. Netw. Model. Anal. Heal. Inform. Bioinf. (2019). https://doi.org/10.1007/s13721-019-0198-0
N. Singh, N. Adlakha, Nonlinear dynamic modeling of 2-dimensional interdependent calcium and inositol 1,4,5-trisphosphate in cardiac myocyte. Math. Biol. Bioinforma. 14, 290–305 (2019). https://doi.org/10.17537/2019.14.290
N. Singh, N. Adlakha, Three dimensional coupled reaction-diffusion modeling of calcium and inositol 1,4,5-trisphosphate dynamics in cardiomyocytes. RSC Adv. 9, 42459–42469 (2019). https://doi.org/10.1039/c9ra06929a
S.G. Tewari, K.R. Pardasani, Modeling effect of sodium pump on calcium oscillations in neuron cells. J. Multiscale Model. 04, 1250010 (2012). https://doi.org/10.1142/s1756973712500102
V. Tewari, S. Tewari, K.R. Pardasani, A model to study the effect of excess buffers and Na+ ions on Ca2+ diffusion in neuron cell. World Acad. Sci. Eng. Technol. 76, 41–46 (2011). https://doi.org/10.5281/zenodo.1054988
S.G. Tewari, K.R. Pardasani, Finite element model to study two dimensional unsteady state cytosolic calcium diffusion in presence of excess buffers. IAENG Int. J. Appl. Math. 40, 1–5 (2010)
S. Tewari, K.R. Pardasani, Finite difference model to study the effects of Na+ influx on cytosolic Ca2+ diffusion. World Acad. Sci. Eng. Technol. 2, 670–675 (2008)
N. Manhas, J. Sneyd, K.R. Pardasani, Modelling the transition from simple to complex Ca2+oscillations in pancreatic acinar cells. J. Biosci. 39, 463–484 (2014). https://doi.org/10.1007/s12038-014-9430-3
N. Manhas, K.R. Pardasani, Mathematical model to study IP3 Dynamics dependent calcium oscillations in pancreatic acinar cells. J. Med. Imag. Heal. Inform. 4, 874–880 (2014). https://doi.org/10.1166/jmihi.2014.1333
N. Manhas, K.R. Pardasani, Modelling mechanism of calcium oscillations in pancreatic acinar cells. J. Bioenerg. Biomembr. 46, 403–420 (2014). https://doi.org/10.1007/s10863-014-9561-0
Y.D. Jagtap, N. Adlakha, Simulation of buffered advection diffusion of calcium in a hepatocyte cell. Math. Biol. Bioinforma. 13, 609–619 (2018). https://doi.org/10.17537/2018.13.609
Y. Jagtap, N. Adlakha, Finite volume simulation of two dimensional calcium dynamics in a hepatocyte cell involving buffers and fluxes. Commun. Math. Biol. Neurosci. 2018, 1–16 (2018). https://doi.org/10.28919/cmbn/3689
Y. Jagtap, N. Adlakha, Numerical study of one-dimensional buffered advection–diffusion of calcium and IP 3 in a hepatocyte cell. Netw. Model. Anal. Heal. Inform. Bioinforma. 8, 1–9 (2019). https://doi.org/10.1007/s13721-019-0205-5
S. Panday, K.R. Pardasani, Finite element model to study effect of advection diffusion and Na+/Ca2+ exchanger on Ca2+ distribution in oocytes. J. Med. Imag. Heal. Inform. 3, 374–379 (2013). https://doi.org/10.1166/jmihi.2013.1184
P.A. Naik, K.R. Pardasani, One dimensional finite element model to study calcium distribution in oocytes in presence of VGCC, RyR and buffers. J. Med. Imaging Heal. Inform. 5, 471–476 (2015). https://doi.org/10.1166/jmihi.2015.1431
P.A. Naik, K.R. Pardasani, Two dimensional finite element model to study calcium distribution in oocytes. J. Multiscale Model. 06, 1450002 (2015). https://doi.org/10.1142/s1756973714500024
P.A. Naik, K.R. Pardasani, Finite element model to study calcium distribution in oocytes involving voltage gated Ca2+ channel, ryanodine receptor and buffers, Alexandria. J. Med. 52, 43–49 (2016). https://doi.org/10.1016/j.ajme.2015.02.002
P.A. Naik, K.R. Pardasani, 2D finite-element analysis of calcium distribution in oocytes. Netw. Model. Anal. Heal. Inform. Bioinforma. 7, 1–11 (2018). https://doi.org/10.1007/s13721-018-0172-2
P.A. Naik, K.R. Pardasani, Three-dimensional finite element model to study effect of RyR calcium channel, ER leak and SERCA pump on calcium distribution in oocyte cell. Int. J. Comput. Methods. 16, 1–19 (2019). https://doi.org/10.1142/S0219876218500913
B.K. Jha, N. Adlakha, M.N. Mehta, Finite volume model to study the effect of buffer on cytosolic Ca2+ advection diffusion. World Acad. Sci. Eng. Technol. 51, 982–985 (2011)
B. Jha, N. Adlakha, M. Mehta, Solution of advection diffusion equation arising in cytosolic calcium concentration distribution. Int. J. Appl. Math Mech. 7: 72–79 (2011). http://ijamm.bc.cityu.edu.hk/ijamm/outbox/Y2011V7N6P72C98630924.pdf
B.K. Jha, N. Adlakha, M.N. Mehta, Two-dimensional finite element model to study calcium distribution in astrocytes in presence of VGCC and excess buffer. Int. J. Model. Simulat. Sci. Comput. (2013). https://doi.org/10.1142/S1793962312500304
B.K. Jha, N. Adlakha, M.N. Mehta, Two-dimensional finite element model to study calcium distribution in astrocytes in presence of excess buffer. Int. J. Biomath. 7, 1–11 (2014). https://doi.org/10.1142/S1793524514500314
A. Tripathi, N. Adlakha, Closed form solution to problem of calcium diffusion in cylindrical shaped neuron cell. World Acad. Sci. Eng. Technol. 80, 739–742 (2011)
A. Tripathi, N. Adlakha, Two dimensional coaxial circular elements in FEM to study calcium diffusion in neuron cells. Appl. Math. Sci. 6, 455–466 (2012)
A. Jha, N. Adlakha, Two-dimensional finite element model to study unsteady state Ca2+ diffusion in neuron involving ER LEAK and SERCA. Int. J. Biomath. (2015). https://doi.org/10.1142/S1793524515500023
A. Jha, N. Adlakha, Analytical solution of two dimensional unsteady state problem of calcium diffusion in a neuron cell. J. Med. Imag. Heal. Inform. 4, 547–553 (2014). https://doi.org/10.1166/jmihi.2014.1282
H. Bhardwaj, N. Adlakha, Radial basis function based differential quadrature approach to study reaction diffusion of Ca2+ in T Lymphocyte. Int. J. Computat. Methods. (2022). https://doi.org/10.1142/S0219876222500591
A. Pawar, K. Raj Pardasani, Effects of disorders in interdependent calcium and IP3 dynamics on nitric oxide production in a neuron cell. Eur. Phys. J. Plus. 137, 543 (2022). https://doi.org/10.1140/EPJP/S13360-022-02743-2
A. Pawar, K.R. Pardasani, Effect of disturbances in neuronal calcium and IP3 dynamics on β-amyloid production and degradation. Cogn. Neurodynamics 2022, 1–18 (2022). https://doi.org/10.1007/S11571-022-09815-0
A. Pawar, K.R. Pardasani, Simulation of disturbances in interdependent calcium and β-amyloid dynamics in the nerve cell. Eur. Phys. J. Plus. 137, 1–23 (2022). https://doi.org/10.1140/EPJP/S13360-022-03164-X
A. Pawar, K.R. Pardasani, Study of disorders in regulatory spatiotemporal neurodynamics of calcium and nitric oxide. Cogn. Neurodynamic. (2022). https://doi.org/10.1007/s11571-022-09902-2
Y.X. Li, R. Bertram, J. Rinzwl, Modeling N-Methyl-D-Aspartate-induced bursting in dopamine neurons. Neuroscience. 71, 397–410 (1996)
C.C. Canavier, Sodium dynamics underlying burst firing and putative mechanisms for the regulation of the firing pattern in midbrain dopamine neurons: A computational approach. J. Comput. Neurosci. 6, 49–69 (1999). https://doi.org/10.1023/A:1008809000182
B. Amini, J.W. Clark, C.C. Canavier, N. Orleans, Calcium dynamics underlying pacemaker-like and burst firing oscillations in midbrain dopaminergic neurons: A computational study. J. Neurophysiol. 82, 49–62 (2019)
G.W. Zamponi, J. Striessnig, A. Koschak, A.C. Dolphin, The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 67, 821–870 (2015). https://doi.org/10.1124/pr.114.009654
M.E. Soden, G.L. Jones, C.A. Sanford, A.S. Chung, A.D. Güler, C. Chavkin, R. Luján, L.S. Zweifel, Disruption of dopamine neuron activity pattern regulation through selective expression of a human KCNN3 mutation. Neuron 80, 997–1009 (2013). https://doi.org/10.1016/j.neuron.2013.07.044
L. Zhang, Y. Liu, X. Chen, Carbachol induces burst firing of dopamine cells in the ventral tegmental area by promoting calcium entry through L-type channels in the rat. J. Physiol. 568, 469–481 (2005). https://doi.org/10.1113/jphysiol.2005.094722
A.A. Grace, B.S. Bunney, The control of firing pattern in nigral dopamine neurons: Burst firing. J. Neurosci. 4, 2877–2890 (1984). https://doi.org/10.1523/jneurosci.04-11-02877.1984
L.A. Sombers, M. Beyene, R.M. Carelli, R.M. Wightman, Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J. Neurosci. 29, 1735–1742 (2009). https://doi.org/10.1523/JNEUROSCI.5562-08.2009
T. Nakano, T. Doi, J. Yoshimoto, K. Doya, A kinetic model of dopamine- and calcium-dependent striatal synaptic plasticity. PLoS Comput. Biol. (2010). https://doi.org/10.1371/journal.pcbi.1000670
M.D. Clinic, Ourette’s 345, 1184–1192 (2001)
E.V. Mosharov, K.E. Larsen, E. Kanter, K.A. Phillips, K. Wilson, Y. Schmitz, D.E. Krantz, K. Kobayashi, R.H. Edwards, D. Sulzer, Article interplay between cytosolic dopamine, calcium, and a-synuclein causes selective death of substantia Nigra neurons. Neuron 62, 218–229 (2009). https://doi.org/10.1016/j.neuron.2009.01.033
T. Yamada, P.L. Mcgeer, K.G. Baimbridge, E.G. Mcgeer, Relative sparing in Parkinson‘s disease of substantia nigra dopamine neurons containing calbindin-D2sK. Brain Res. 526, 303–307 (1990)
M.K. Sanghera, J. Zamora, D.C. German, Calbindin-D 28k-containing neurons in the human hypothalamus: relationship to dopaminergic neurons. Brain Res. 4, 375–381 (1995)
Y. Tabata, Y. Imaizumi, M. Sugawara, T. Andoh-Noda, S. Banno, M.C. Chai, T. Sone, K. Yamazaki, M. Ito, K. Tsukahara, H. Saya, N. Hattori, J. Kohyama, H. Okano, T-type calcium channels determine the vulnerability of dopaminergic neurons to mitochondrial stress in familial Parkinson disease. Stem. Cell Reports. 11, 1171–1184 (2018). https://doi.org/10.1016/j.stemcr.2018.09.006
A. Mcmahon, B.S. Wong, A.M. Iacopino, M.C. Ng, S. Chi, D.C. German, Calbindin-D 28 k buffers intracellular calcium and promotes resistance to degeneration in PC12 cells. Mol. Brain Res. 54, 56–63 (1998)
H. Joshi, B.K. Jha, Fractionally delineate the neuroprotective function of calbindin- D 28 k in Parkinson’s disease. Int. J. Biomath. 11, 1–19 (2018). https://doi.org/10.1142/S1793524518501036
J. Wagner, C.F. Fall, F. Hong, C.E. Sims, N.L. Allbritton, R.A. Fontanilla, I.I. Moraru, L.M. Loew, R. Nuccitelli, A wave of IP3 production accompanies the fertilization Ca2+ wave in the egg of the frog, Xenopus laevis: Theoretical and experimental support. Cell Calcium 35, 433–447 (2004). https://doi.org/10.1016/j.ceca.2003.10.009
D. Tello-Bravo, A mathematical model of dopamine neurotransmission. Gastron. Ecuatoriana y Tur. Local. 1, 5–24 (1967)
G. Dupont, S. Swillens, C. Clair, T. Tordjmann, L. Combettes, Hierarchical organization of calcium signals in hepatocytes: From experiments to models. Biochim. Biophys. Acta-Mol. Cell Res. 1498, 134–152 (2000). https://doi.org/10.1016/S0167-4889(00)00090-2
S.H. Walters, I.M. Taylor, Z. Shu, A.C. Michael, A novel restricted diffusion model of evoked dopamine. ACS Chem. Neurosci. 5, 776–783 (2014). https://doi.org/10.1021/cn5000666
S.A. Brown, F. Morgan, J. Watras, L.M. Loew, Analysis of phosphatidylinositol-4,5-bisphosphate signaling in cerebellar Purkinje spines. Biophys. J. 95, 1795–1812 (2008). https://doi.org/10.1529/biophysj.108.130195
T. Öziş, E.N. Aksan, A. Özdeş, A finite element approach for solution of Burgers’ equation. Appl. Math. Comput. 139, 417–428 (2003). https://doi.org/10.1016/S0096-3003(02)00204-7
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Model equations description
For [Ca2+] and dopamine mechanisms, the shape functions for each element are expressed as:
The nodal conditions in Eq. (19) yields,
where
By Eq. (21), we have
And
Adding \(q^{(e)}\) and \(r^{(e)}\) from Eq. (23) in (19) yields,
For Eqs. (1 and 8), the discretized form is provided as,
The integrals \(I_{1}^{(e)}\) and \(I_{2}^{(e)}\) can be expressed as,
where
Now,
The various parameters are determined by linearization of nonlinear [Ca2+] and dopamine mechanisms \(\alpha , \, \gamma , \, \kappa , \, \eta , \, \mu_{1} , \, \mu_{2} , \, \beta_{1} ,{\text{ and }}\beta_{2}\). The equations are analyzed, and boundary conditions are added to get the following system of equations.
where
The time derivatives in the finite element techniques were solved by Crank–Nicolson scheme for system matrices (A and B) and system vectors (F).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pawar, A., Pardasani, K.R. Computational model of calcium dynamics-dependent dopamine regulation and dysregulation in a dopaminergic neuron cell. Eur. Phys. J. Plus 138, 30 (2023). https://doi.org/10.1140/epjp/s13360-023-03691-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-023-03691-1