Abstract
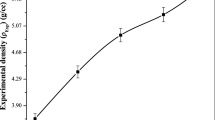
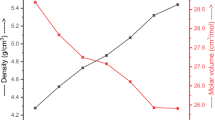
Fast quenching was used to create glasses in the xFe2O3·(40x)Ag2O·60P2O5 system (0 ≤ x ≤ 20 mol%). Fourier transform infrared spectrum (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM) were used to investigate the structure of the glasses. A gradual increase in Fe2O3 content leads to an increase in P–O–Fe bonds, which increases the durability of the glass structure. The Hardness number (Hv) of the investigated glasses increases with increasing Fe2O3 content. The measured density decreases, and the calculated molar volume increases with increasing iron oxide concentrations. The mass attenuation coefficient was calculated using the WinXCOM program after being measured experimentally. The experimental and theoretical values are in good agreement. The gamma-ray shielding parameter HVL was calculated to understand the radiation shielding performances of the investigated glasses. The results show that as the Fe2O3 content increases, the mass attenuation coefficient (μm) decreases. The glass with the lowest HVL values contains 5 mol% Fe2O3.













Similar content being viewed by others
References
Y.M. Lai, X.F. Liang, S.Y. Yang, J.X. Wang, L.H. Cao, B. Dai, Raman and FTIR spectra of iron phosphate glasses containing cerium. J. Mol. Struct. 992(1–3), 84–88 (2011)
C.W. Kim, C.S. Ray, D. Zhu, D.E. Day, D. Gombert, A. Aloy, A. Moguš-Milanković, M. Karabulut, Chemically durable iron phosphate glasses for vitrifying sodium bearing waste (SBW) using conventional and cold crucible induction melting (CCIM) techniques. J. Nucl. Mater. 322(2–3), 152–164 (2003)
M. El-Desoky, A. Al-Shahrani, Mixed electronic–ionic conductivity in semiconducting CaO–PbO–Fe2O3–P2O5 glasses. Physica B 371(1), 95–99 (2006)
Q. Shi, Y. Yue, Y. Qu, S. Liu, G.A. Khater, L. Zhang, J. Zhao, J. Kang, Structure and chemical durability of calcium iron phosphate glasses doped with La2O3 and CeO2. J. Non-Cryst. Solids 516, 50–55 (2019)
K.B. Richard, Review: the structure of simple phosphate glasses. J. Non-Cryst. Solids 263, 1–28 (2000)
G. El Damrawi, A. Hassan, A. Shahboub, Chemical durability and structure of Al2O3–Ag2O–P2O5 glasses. Appl. Phys. A 126(4), 1–7 (2020)
M. Laourayed, M. El Moudane, M. Khachani, M. Boudalia, A. Guenbour, A. Bellaouchou, M. Tabyaoui, Effect of the Bi2O3 on the thermal, structural and chemical durability of some bismuth niobium phosphate glasses. Mater. Today: Proc. 13, 974–981 (2019)
B. Sales, L. Boatner, Lead-iron phosphate glass: a stable storage medium for high-level nuclear waste. Science 226(4670), 45–48 (1984)
S. Azianty, A. Yahya, M. Halimah, Effects of Fe2O3 replacement of ZnO on elastic and structural properties of 80TeO2–(20–x) ZnO–x Fe2O3 tellurite glass system. J. Non-Cryst. Solids 358(12–13), 1562–1568 (2012)
D. Rusu, I. Ardelean, Structural studies of Fe2O3–Bi2O3–CdO glass system. Mater. Res. Bull. 43(7), 1724–1730 (2008)
A. Ghosh, Memory switching in bismuth-vanadate glasses. J. Appl. Phys. 64(5), 2652–2655 (1988)
A. El-Taher, A.M. Ali, Y.B. Saddeek, R. Elsaman, H. Algarni, K. Shaaban, T.Z. Amer, Gamma ray shielding and structural properties of iron alkali alumino-phosphate glasses modified by PbO. Radiat. Phys. Chem. 165, 108403 (2019)
C.W. Kim, D.E. Day, Immobilization of Hanford LAW in iron phosphate glasses. J. Non-Cryst. Solids 331(1–3), 20–31 (2003)
S. Reis, M. Karabulut, D. Day, Chemical durability and structure of zinc–iron phosphate glasses. J. Non-Cryst. Solids 292(1–3), 150–157 (2001)
M. Karabulut, G.K. Marasinghe, C.S. Ray, D.E. Day, O. Ozturk, G.D. Waddill, X-ray photoelectron and Mössbauer spectroscopic studies of iron phosphate glasses containing U, Cs and Bi. J. Non-Cryst. Solids 249(2–3), 106–116 (1999)
Y. Elmahroug, B. Tellili, C. Souga, Determination of total mass attenuation coefficients, effective atomic numbers and electron densities for different shielding materials. Ann. Nucl. Energy 75, 268–274 (2015)
S.M. Hsu, S.W. Yung, R.K. Brow, W.L. Hsu, C.C. Lu, F.B. Wu, S.H. Ching, Effect of silver concentration on the silver-activated phosphate glass. Mater. Chem. Phys. 123(1), 172–176 (2010)
I.O. Olarinoye, S. Alomairy, C. Sriwunkum, M.S. Al-Buriahi, Effect of Ag2O/V2O5 substitution on the radiation shielding ability of tellurite glass system via XCOM approach and FLUKA simulations. Phys. Scr. 96(6), 065308 (2021)
Y. Miyamoto, Y. Takei, H. Nanto, T. Kurobori, A. Konnai, T. Yanagida, A. Yoshikawa, Y. Shimotsuma, M. Sakakura, K. Miura, K. Hirao, Radiophotoluminescence from silver-doped phosphate glass. Radiat. Meas. 46(12), 1480–1483 (2011)
M. Sayyed, M. Rashad, Y. Rammah, Impact of Ag2O on linear, nonlinear optical and gamma-ray shielding features of ternary silver vanadio-tellurite glasses: TeO2–V2O5–Ag2O. Ceram. Int. 46(14), 22964–22972 (2020)
U. Hoppe, G. Walter, A. Barz, D. Stachel, A.C. Hannon, The PO bond lengths in vitreous probed by neutron diffraction with high real-space resolution. J. Phys.: Condens. Matter 10(2), 261 (1998)
G. El-Damrawi, A. Hassan, H. Doweidar, A. Shaboub, Structural studies on Ag2O-P2O5 glasses. New J. Glass Ceram. 7(03), 77 (2017)
D.E. Day, Z. Wu, C.S. Ray, P. Hrma, Chemically durable iron phosphate glass waste forms. J. Non-Cryst. Solids 241(1), 1–2 (1998)
B.C. Sales, M.M. Abraham, J.B. Bates, L.A. Boatner, Structural properties of lead-iron phosphate glasses. J. Non-Cryst. Solids 71(1–3), 103–112 (1985)
S.V. Stefanovsky, O.I. Stefanovsky, M.I. Kadyko, I.A. Presniakov, B.F. Myasoedov, The effect of Fe2O3 substitution for Al2O3 on the phase composition and structure of sodium–aluminum–iron phosphate glasses. J. Non-Cryst. Solids 425, 138–145 (2015)
G.K. Marasinghe, M. Karabulut, C.S. Ray, D.E. Day, D.K. Shuh, P.G. Allen, M.L. Saboungi, M. Grimsditch, D. Haeffner, Properties and structure of vitrified iron phosphate nuclear wasteforms. J. Non-Cryst. Solids 263, 146–154 (2000)
C.S. Ray, X. Fang, M. Karabulut, G.K. Marasinghe, D.E. Day, Effect of melting temperature and time on iron valence and crystallization of iron phosphate glasses. J. Non-Cryst. Solids 249(1), 1–16 (1999)
J.S. Alzahrani, M.A. Alothman, C. Eke, H. Al-Ghamdi, D.A. Aloraini, M.S. Al-Buriahi, Simulating the radiation shielding properties of TeO2–Na2O–TiO glass system using PHITS Monte Carlo code. Comput. Mater. Sci. 196, 110566 (2021)
I. Kebaili, S. Znaidia, J.S. Alzahrani, M.A. Alothman, I. Boukhris, I.O. Olarinoye, C. Mutuwong, M.S. Al-Buriahi, Ge20Se80-xBix (x≤ 12) chalcogenide glasses for infrared and gamma sensing applications: structural, optical and gamma attenuation aspects. J. Mater. Sci.: Mater. Electron. 32, 15509–15522 (2021)
D.F. Jackson, D.J. Hawkes, X-ray attenuation coefficients of elements and mixtures. Phys. Rep. 70(3), 169–233 (1981)
L. Gerward, N. Guilbert, K.B. Jensen, H. Leving, WinXCom–a program for calculating X-ray attenuation coefficients. Radiat. Phys. Chem. 71, 653–654 (2004)
Y. Moustafa, K. El-Egili, Infrared spectra of sodium phosphate glasses. J. Non-Cryst. Solids 240(1–3), 144–153 (1998)
J.H. Hubbell, Photon mass attenuation and energy-absorption coefficients. Int. J. Appl. Radiat. Isot. 33(11), 1269–1290 (1982)
C. Dayanand, G. Bhikshamaiah, V.J. Tyagaraju, M. Salagram, A.K. Murthy, Structural investigations of phosphate glasses: a detailed infrared study of the x(PbO)-(1–x) P2O5 vitreous system. J. Mater. Sci. 31(8), 1945–1967 (1996)
K. El-Egili, H. Doweidar, Y.M. Moustafa, I. Abbas, Structure and some physical properties of PbO–P2O5 glasses. Physica B 339(4), 237–245 (2003)
A.M. Efimov, IR fundamental spectra and structure of pyrophosphate glasses along the 2ZnO· P2O5–2Me2O· P2O5 join (Me being Na and Li). J. Non-Cryst. Solids 209(3), 209–226 (1997)
N. Vedeanu, D. Magdas, R. Stefan, Structural modifications induced by addition of copper oxide to lead–phosphate glasses. J. Non-Cryst. Solids 358(23), 3170–3174 (2012)
J.P. Malugani, R. Mercier, Vibrational properties of and short range order in superionic glasses AgPO3–AgX (X = I, Br, Cl). Solid State Ionics 13(4), 293–299 (1984)
M.H. Misbah, H. Doweidar, K. El-Egili, G. El-Damrawi, M. El-Kemary, Structure and some properties of xBaO· (50–x) PbO· 50P2O5 glasses. J. Non-Cryst. Solids 534, 119945 (2020)
E.L. Kamitsos, J.A. Kapoutsis, G.D. Chryssikos, J.M. Hutchinson, A.J. Pappin, Infrared study of AgI containing superionic glasses. Phys. Chem. Glasses 36(3), 141–149 (1995)
D. Corbridge, E. Lowe, The infra-red spectra of some inorganic phosphorus compounds. J. Chem. Soc. (Resumed) (1954). https://doi.org/10.1039/JR9540000493
H. Liu, T. Chin, S. Yung, FTIR and XPS studies of low-melting PbO-ZnO-P2O2 glasses. Mater. Chem. Phys. 50(1), 1–10 (1997)
M. Abid, M. Et-Tabirou, M. Taibi, Structure and DC conductivity of lead sodium ultraphosphate glasses. Mater. Sci. Eng. B 97(1), 20–24 (2003)
J.O. Byun, B.H. Kim, K.S. Hong, H.J. Jung, S.W. Lee, A.A. Izyneev, Properties and structure of RO–Na2O–Al2O3–P2O5 (R = Mg, Ca, Sr, Ba) glasses. J. Non-Cryst. Solids 190(3), 288–295 (1995)
S.T. Reis, A. Moguš-Milanković, V. Ličina, J.B. Yang, M. Karabulut, D.E. Day, R.K. Brow, Iron redox equilibrium, structure and properties of zinc iron phosphate glasses. J. Non-Cryst. Solids 353(2), 151–158 (2007)
D. Ilieva, B. Jivov, G. Bogachev, C. Petkov, I. Penkov, Y. Dimitriev, Infrared and Raman spectra of Ga2O3–P2O5 glasses. J. Non-Cryst. Solids 283(1–3), 195–202 (2001)
Y.M. Moustafa, K. El-Egili, H. Doweidar, I. Abbas, Structure and electric conduction of Fe2O3–P2O5 glasses. Physica B 353(1–2), 82–91 (2004)
G. El-Damrawi, A. Hassan, A. Shahboub, 31P and 27Al nuclear magnetic resonance studies on silver phosphate glasses. Magn. Resonance Solids 20(2), 1–10 (2018)
D. Toloman, A.R. Biris, D. Maniu, I. Bratu, L.M. Giurgiu, A.S. Biris, I. Ardelean, Phosphate glassy network depolymerization induced by CaO doping. Part. Sci. Technol. 28(3), 226–235 (2010)
M. Lu, F. Wang, Q. Liao, K. Chen, J. Qin, S. Pan, FTIR spectra and thermal properties of TiO2-doped iron phosphate glasses. J. Mol. Struct. 1081, 187–192 (2015)
Y. Lai, X. Liang, G. Yin, S. Yang, J. Wang, H. Zhu, H. Yu, Infrared spectra of iron phosphate glasses with gadolinium oxide. J. Mol. Struct. 1004(1–3), 188–192 (2011)
H. Doweidar, Y.M. Moustafa, K. El-Egili, I. Abbas, Infrared spectra of Fe2O3–PbO–P2O5 glasses. Vib. Spectrosc. 37(1), 91–96 (2005)
P. Bergo, S.T. Reis, W.M. Pontuschka, J.M. Prison, C.C. Motta, Dielectric properties and structural features of barium-iron phosphate glasses. J. Non-Cryst. Solids 336(3), 159–164 (2004)
K. Joseph, K.G. Kutty, P. Chandramohan, P.V. Rao, Studies on the synthesis and characterization of cesium-containing iron phosphate glasses. J. Nucl. Mater. 384(3), 262–267 (2009)
P. Stoch, W. Szczerba, W. Bodnar, M. Ciecinska, A. Stoch, E. Burkel, Structural properties of iron-phosphate glasses: spectroscopic studies and ab initio simulations. Phys. Chem. Chem. Phys. 16(37), 19917–19927 (2014)
T. Okura, T. Miyachi, H. Monma, Properties and vibrational spectra of magnesium phosphate glasses for nuclear waste immobilization. J. Eur. Ceram. Soc. 26(4–5), 831–836 (2006)
J.E. Pemberton, L. Latifzadeh, J.P. Fletcher, S.H. Risbud, Raman spectroscopy of calcium phosphate glasses with varying calcium oxide modifier concentrations. Chem. Mater. 3(1), 195–200 (1991)
X. Yu, D.E. Day, G.J. Long, R.K. Brow, Properties and structure of sodium-iron phosphate glasses. J. Non-Cryst. Solids 215(1), 21–31 (1997)
P.Y. Shih, Properties and FTIR spectra of lead phosphate glasses for nuclear waste immobilization. Mater. Chem. Phys. 80(1), 299–304 (2003)
R.K. Brow, D.R. Tallant, S.T. Myers, C.C. Phifer, The short-range structure of zinc polyphosphate glass. J. Non-Cryst. Solids 191(1–2), 45–55 (1995)
X. Liang, H. Li, C. Wang, H. Yu, Z. Li, S. Yang, Physical and structural properties of calcium iron phosphate glass doped with rare earth. J. Non-Cryst. Solids 402, 135–140 (2014)
M. Ganguli, K. Rao, Studies of ternary Li2SO4–Li2O–P2O5 glasses. J. Non-Cryst. Solids 243(2–3), 251–267 (1999)
D.R. Lide, CRC Handbook of Chemistry and Physics, vol. 85 (CRC Press, Boca Raton, 2004)
W. Ahmina, M. El Moudane, A. Shaim, M. Zriouil, M. Taibi, Chemical durability, electrical and dielectric properties of the ternary system (50–x)K2O–xMnO–50P2O5 phosphate glasses. Mater. Today: Proc. 13, 466–473 (2019)
P. Shih, T. Chin, Effect of redox state of copper on the properties of P2O5–Na2O–CuO glasses. Mater. Chem. Phys. 60(1), 50–57 (1999)
M.S. Al-Buriahi, E.M. Bakhsh, B. Tonguc, S.B. Khan, Mechanical and radiation shielding properties of tellurite glasses doped with ZnO and NiO. Ceram. Int. 46(11), 19078–19083 (2020)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahboub, A., El Damrawi, G. & Saleh, A. A new focus on the role of iron oxide in enhancing the structure and shielding properties of Ag2O–P2O5 glasses. Eur. Phys. J. Plus 136, 947 (2021). https://doi.org/10.1140/epjp/s13360-021-01948-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-021-01948-1