Abstract.
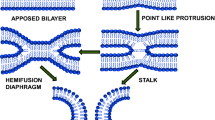
Proteins involved in membrane fusion, such as SNARE or influenza virus hemagglutinin, share the common function of pulling together opposing membranes in closer contact. The reduction of inter-membrane distance can be sufficient to induce a lipid transition phase and thus fusion. We have used functionalized lipids bearing DNA bases as head groups incorporated into giant unilamellar vesicles in order to reproduce the reduction of distance between membranes and to trigger fusion in a model system. In our experiments, two vesicles were isolated and brought into adhesion by the mean of micromanipulation; their evolution was monitored by fluorescence microscopy. Actual fusion only occurred in about 5% of the experiments. In most cases, a state of “hemifusion” is observed and quantified. In this state, the outer leaflets of both vesicles’ bilayers merged whereas the inner leaflets and the aqueous inner contents remained independent. The kinetics of the lipid probes redistribution is in good agreement with a diffusion model in which lipids freely diffuse at the circumference of the contact zone between the two vesicles. The minimal density of bridging structures, such as stalks, necessary to explain this redistribution kinetics can be estimated.
Similar content being viewed by others
References
T. Weber et al. , Cell 92, 759 (1998).
S.M. Small, The Physical Chemistry of Lipids. From Alkanes to Phospholipids (Plenum Publishing Corporation, New York, 1986).
J.A. McNew et al. , J. Cell Biol. 150, 105 (2000).
W. Almers, F.W. Tse, Neuron 4, 813 (1990).
P. Durrer et al. , J. Biol. Chem. 271, 13417 (1996).
R. Jahn, T.C. Südhof, Annu. Rev. Biochem. 68, 863 (1999).
L. Yang, H.W. Huang, Science 297, 1877 (2002).
B.R. Lentz, J.K. Lee, Mol. Membr. Biol. 16, 279 (1999).
W. Helfrich, R.-M. Servuss, Nuovo Cimento D 3, 137 (1984).
J. Israelachvili, Intermolecular and Surface Forces (Academic Press, San Diego, 1985).
L. Lis et al. , Biophys. J. 37, 667 (1982).
R.P. Rand, V.A. Parsegian, Biochim. Biophys. Acta 988, 351 (1989).
F. Pincet, L. Lebeau, S. Cribier, Eur. Biophys. J. 30, 91 (2000).
F. Pincet et al. , Phys. Rev. Lett. 73, 2780 (1994).
E. Perez et al. , Eur. Phys. J. B 6, 1 (1998).
R.L. Ornberg, T.S. Reese, J. Cell Biol. 90, 40 (1981).
M.M. Kozlov, L.V. Chernomordik, Biophys. J. 75, 1384 (1998).
P.I. Kuzmin, J. et al. , Proc. Natl. Acad. Sci. USA 98, 7325 (2001).
L. Lebeau et al. , Chem. Phys. Lipids 62, 93 (1992).
M.I. Angelova et al. , Progr. Colloid Polym. Sci. 89, 127 (1992).
L. Mathivet, S. Cribier, P.F. Devaux, Biophys. J. 70, 1112 (1996).
R. Kwok, E. Evans, Biophys. J. 35, 637 (1981).
M. Arrio-Dupont et al. , Biophys. J. 70, 2327 (1996).
E. Evans et al. , Science 273, 933 (1996).
T.J. McIntosh, D. Magid, in Phospholipids Handbook, edited by G. Cevc (Dekker, New York, 1993).
C. Helm, J.N. Israelachvili, P. Mc Guiggan, Biochemistry 31, 1794 (1992).
T. Kuhl et al. , Langmuir 12, 3003 (1996).
S.A. Safran, T.L. Kuhl, J.N. Israelachvili, Biophys. J. 81, 659 (2001).
L. Chernomordik et al. , Biophys. J. 69, 922 (1995).
D. Siegel, Biophys. J. 65, 2124 (1993).
E. Evans, D. Needham, J. Phys. Chem. 91, 4219 (1987)
R.J. Rubin, Y. Chen, Biophys. J. 58, 1157 (1990).
L.K. Tamm, H.M. McConnell, Biophys. J. 47, 105 (1985).
H.C. Berg, E.M. Purcell, Biophys. J. 20, 193 (1977).
V. Marchi-Artzner et al. , J. Phys. Chem. 100, 13844 (1996).
F.S. Cohen et al. , J. Cell Biol. 98, 1054 (1984).
G.B. Melikyan, J.M. White, F.S. Cohen, J. Cell Biol. 131, 679 (1995).
Author information
Authors and Affiliations
Corresponding author
Additional information
Received: 26 May 2004, Published online: 3 August 2004
PACS:
87.16.Dg Subcellular structure and processes: Membranes, bilayers, and vesicles - 87.15.Vv Biomolecules: structure and physical properties: Diffusion - 64.70.Nd Structural transitions in nanoscale materials
Rights and permissions
About this article
Cite this article
Heuvingh, J., Pincet, F. & Cribier, S. Hemifusion and fusion of giant vesicles induced by reduction of inter-membrane distance. Eur. Phys. J. E 14, 269–276 (2004). https://doi.org/10.1140/epje/i2003-10151-2
Issue Date:
DOI: https://doi.org/10.1140/epje/i2003-10151-2