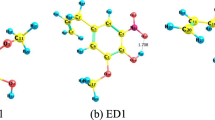
Abstract
Probucol (PB) is a lipid-regulating agent with powerful antioxidant, anti-inflammatory and anti-atherogenic effects. In this paper, based on density functional theory (DFT), B3LYP/def2tzvp functional and basis set are used to optimize the structure of PB molecule, and the vibration attribution analysis is carried out. On the basis of optimization, the first 40 excited states of molecule in anhydrous ethanol were calculated by time-density functional theory (TD-DFT). Then UV spectrum and electron–hole diagrams are drawn to analyze the excited state properties. Finally, the antioxidant mechanism of PB was theoretically analyzed by predicting the active site of PB molecule. This study has two purposes: first, to verify the experimental spectra, and second, to provide basic data for the properties of PB molecule, and provide theoretical reference for its antioxidant mechanism in clinical medicine, as well as antioxidant detection in food and care products.
Graphic abstract










Similar content being viewed by others
Data Availability Statement
This manuscript has associated data in a data repository. [Author’s Comment: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request].
References
A.M. Gotto Jr., Dyslipidemia and atherosclerosisA forecast of pharmaceutical approaches. Circulation 87(4), 1154–1159 (1993)
M. Morita, Y. Naito et al., Inhibition of plasma lipid oxidation induced by peroxyl radicals, peroxynitrite, hypochlorite, 15-lipoxygenase, and singlet oxygen by clinical drugs. Bioorg. Med. Chem. Lett. 26(22), 5411–5417 (2016). https://doi.org/10.1016/j.bmcl.2016.10.033
A. Mooranian, N. Zamani et al., Stability and biological testing of taurine-conjugated bile acid antioxidant microcapsules for diabetes treatment. Ther. Deliv. 10(2), 99–106 (2019). https://doi.org/10.4155/tde-2018-0034
M. Shichiri, N. Ishida et al., Probucol induces the generation of lipid peroxidation products in erythrocytes and plasma of male cynomolgus macaques. J. Clin. Biochem. Nutr. 64(2), 129–142 (2018). https://doi.org/10.3164/jcbn.18-7
Y. Shizuya, M. Daisaku et al., Rationale and design of the PROSPECTIVE trial: probucol trial for secondary prevention of atherosclerotic events in patients with prior coronary heart disease. J. Atheroscler. Thromb. 23(6), 746–756 (2016)
H. Zhu, X. Jin et al., Probucol protects against atherosclerosis through lipid-lowering and suppressing immune maturation of CD11c+ dendritic cells in STZ-induced diabetic LDLR−/− mice. J. Cardiovasc. Pharmacol. 65(6), 620–627 (2015). https://doi.org/10.1097/FJC.0000000000000234
M. Pallebage-Gamarallage, R. Takechi et al., Pharmacological modulation of dietary lipid-induced cerebral capillary dysfunction: considerations for reducing risk for Alzheimer’s disease. Crit. Rev. Clin. Lab. Sci. 53(3), 166–183 (2015). https://doi.org/10.3109/10408363.2015.1115820
J.C.L. Mamo, V. Lam et al., Sodium alginate capsulation increased brain delivery of probucol and suppressed neuroinflammation and neurodegeneration. Therap. Deliv. 9(10), 703–709 (2018). https://doi.org/10.4155/tde-2018-0033
R.H. Yang, Z.Y. Hu et al., Probucol ameliorates hepatic stellate cell activation and autophagy is associated with farnesoid X receptor. J. Pharmacol. Sci. 139(2), 120–128 (2019). https://doi.org/10.1016/j.jphs.2018.12.005
H.X. Zhang, Y.N. Li et al., Probucol ameliorates EMT and lung fibrosis through restoration of SIRT3 expression. Pulm. Pharmacol. Therap. 57, 101803 (2019). https://doi.org/10.1016/j.pupt.2019.101803
B. Shi, L. Yuan et al., Study on electronic structure and excitation characteristics of cyclo[18]carbon. Chem. Phys. Lett. 741, 136975 (2020). https://doi.org/10.1016/j.cplett.2019.136975
E.N. Nikolaevskaya, P.G. Shangin et al., Easily electroreducible halogen-free germanium complexes with biologically active pyridines. Inorg. Chim. Acta. 495, 119007 (2019). https://doi.org/10.1016/j.ica.2019.119007
S. Kapuciński, M.B. Abdulmojeed et al., Photonic materials derived from the [closo-B10H10]2 anion: tuning photophysical properties in [closo-B10H8-1-X-10-(4-Y-NC5H5)]. Inorg. Chem. Front. 8(4), 1066–1082 (2021). https://doi.org/10.1039/d0qi01353f
B. Mccarthy, R.M. Pearson et al., Structure-property relationships for tailoring phenoxazines as reducing photoredox catalysts. J. Am. Chem. Soc. 140(15), 5088–5101 (2018). https://doi.org/10.1021/jacs.7b12074
X. Blase, I. Duchemin, D. Jacquemin, The Bethe-Salpeter equation in chemistry: relations with TD-DFT, applications and challenges. Chem. Soc. Rev. 47(3), 1022–1043 (2018). https://doi.org/10.1039/c7cs00049a
A. Mavroskoufis, K. Rajes et al., N-heterocyclic carbene catalyzed photoenolization/diels–alder reaction of acid fluorides. Angew. Chem. Int. Ed. 59(8), 3190–3194 (2020). https://doi.org/10.1002/anie.201914456
Y.Z. Lu, L. Feng et al., Construction of a molecular structure model of mild-oxidized Chinese lignite using Gaussian09 based on data from FTIR, solid state C-13-NMR. J. Mol. Modeli. (2018). https://doi.org/10.1007/s00894-018-3677-9
S. Grimme, Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27(15), 1787–1799 (2006). https://doi.org/10.1002/jcc.20495
T. Lu, F.W. Chen, Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33(5), 580–592 (2012). https://doi.org/10.1002/jcc.22885
H. Al-Salami, A. Mooranian et al., An optimized probucol microencapsulated formulation integrating a secondary bile acid (deoxycholic acid) as a permeation enhancer. Drug Des. Dev. Ther. 8, 1673–1683 (2014). https://doi.org/10.2147/DDDT.S68247
X. Y. Zheng, F. Y. Liu, H. J. Guo. Determination of probucol tablets by ultraviolet spectrophotometry. China Pharmaceutical. (11)(2001) 33 (in Chinese)
A. Zl, W.A. Xia et al., Potential optical molecular switch: Lithium@cyclo[18]carbon complex transforming between two stable configurations. Carbon 187, 78–85 (2021). https://doi.org/10.1016/j.carbon.2021.11.005
T. Bahers, A.C. Le, I. Ciofini, A qualitative index of spatial extent in charge-transfer excitation. J. Chem. Theory Comput. 78, 2498–2506 (2011). https://doi.org/10.1021/ct200308m
W. Yang, R.G. Parr, Hardness, softness, and the Fukui function in the electronic theory of metals and catalysis. Proc. Natl. Acad. Sci. U.S.A. 82(20), 6723–6726 (1985). https://doi.org/10.1073/pnas.82.20.6723
C. Morell, A. Grand, A. Toro-Labbe, New dual descriptor for chemical reactivity. J. Phys. Chem. A 109(1), 205–212 (2005). https://doi.org/10.1021/jp046577a
B. Shi, J.C. Yu et al., Study on UV spectrum and antioxidant properties of 3-tert-butyl-4-hydroxyanisole molecule. Russ. J. Phys. Chem. A 95(2), 343–348 (2021). https://doi.org/10.1134/S0036024421020230
Acknowledgements
Thanks to Dr. Lu Tian, Director of the Beijing Kein Research Center for Natural Sciences for his help in the theoretical methods of this paper.
Funding
This work was supported by the National Natural Science Foundation of China [Grant Number 11164004], the Photon Science and Technology Innovation Talent Team of Guizhou Province [Grant Number 20154017].
Author information
Authors and Affiliations
Contributions
ShiQuan Wu: Investigation, Writing—original draft, Software. LiMin Lu, Li Li: Supervision. QiQi Liang, HuaXu Gao: Supervision. TianYu Tang: Supervision. YanLin Tang: Methodology, Data curation, Writing—review & editing, Validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, S., Lu, L., Li, L. et al. Density functional studies of probucol excited states and spectral properties. Eur. Phys. J. D 77, 10 (2023). https://doi.org/10.1140/epjd/s10053-022-00577-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjd/s10053-022-00577-2