Abstract.
Individual amino acid molecules embedded in helium nanodroplets fragment extensively when the beam is ionized by electron bombardment. However, we find that when glycine and tryptophan are picked up right after, or right before, a small amount of water, the mass spectra become significantly altered. For glycine, the detected ions consist almost entirely of intact protonated amino acids, with or without a few water molecules attached. In other words, the presence of water exerts a striking “buffering” effect on the ionization-induced fragmentation. For tryptophan the effect is weaker but also present. In both cases, the hydroxyl group lost upon ionization overwhelmingly comes from the water partner (in strong contrast to the situation observed when amino acids are picked up by neat water clusters). A complementary experiment involving DCl molecules co-embedded with water shows that in this case Cl and/or DCl invariably leave the droplet upon ionization. The observed patterns may be steered by the analytes' dipole moments or by solvation effects.
Similar content being viewed by others
References
J.P. Toennies, A.F. Vilesov, Angew. Chem. Intern. Ed. 43, 2622 (2004)
F. Stienkemeier, K.K. Lehmann, J. Phys. B: At. Mol. Opt. Phys. 39, R127 (2006)
A. Scheidemann, B. Schilling, J.P. Toennies, J. Phys. Chem. 97, 2128 (1993)
B.E. Callicoatt, D.D. Mar, V.A. Apkarian, K.C. Janda, J. Chem. Phys. 105, 7872 (1996)
M. Fárník, J.P. Toennies, J. Chem. Phys. 122, 014307 (2005)
S. Yang, S.M. Brereton, M.D. Wheeler, A.M. Ellis, Phys. Chem. Chem. Phys. 7, 4082 (2005)
F. Huisken, O. Werhahn, A.Yu. Ivanov, S.A. Krasnokutski, J. Chem. Phys. 111, 2978 (1999)
A. Lindinger, J.P. Toennies, A.F. Vilesov, J. Chem. Phys. 110, 1429 (1999)
NIST Mass Spec Data Center, “Mass Spectra”, in NIST Chemistry WebBook, NIST Standard Reference Database No. 69, edited by P. Linstrom, W.G. Mallard (National Institute of Standards and Technology, Gaithersburg, 2005), http://webbook.nist.gov/chemistry
R. Moro, R. Rabinovich, V.V. Kresin, J. Chem. Phys. 123, 074301 (2005)
Glycine was purchased from VWR, deuterated glycine from Icon Services, tryptophan from Aldrich
K. Aflatooni, B. Hitt, G.A. Gallup, P.D. Burrow, J. Chem. Phys. 115, 6489 (2001)
M. Lewerenz, B. Schilling, J.P. Toennies, J. Chem. Phys. 102, 8191 (1995)
R. Fröchtenicht, M. Kaloudis, M. Koch, F. Huisken, J. Chem. Phys. 105, 6128 (1996)
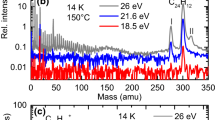
The number of picked-up water molecules is given by a Poisson distribution Toennies:2004, here typically centered at 〈n〉≈3, as evidenced by the maximum at (D2O)2D+ in Figures [SEE TEXT]B and [SEE TEXT]B.
Increased background in the spectrum comes from picked-up fragments of diffusion pump oil abundant in this mass range.
B.D. Kay, Ph.D. dissertation, University of Colorado at Boulder (1982)
R. Moro, R. Rabinovich, V.V. Kresin, J. Chem. Phys. 124, 146102 (2006)
F.J. Lovas, Y. Kawashima, J.U. Grabow, R.D. Suenram, G.T. Fraser, E. Hirota, Astrophys. J. 455, L201 (1995)
M. Yang, P. Senet, C. Van Alsenoy, Int. J. Quantum Chem. 101, 535 (2005)
J.K. Gregory, D.C. Clary, K. Liu, M.G. Brown, R.J. Saykally, Science 275, 814 (1997)
E.W. Kaiser, J. Chem. Phys. 53, 1686 (1970)
R. Antoine, I. Compagnon, D. Rayane, M. Broyer, Ph. Dugourd, G. Breaux, F.C. Hagemeister, D. Pippen, R.R. Hudgins, M.F. Jarrold, Eur. Phys. J. D 20, 583 (2002)
W.K. Lewis, C.M. Lindsay, R.J. Bemish, R.E. Miller, J. Am. Chem. Soc. 127, 7235 (2005)
K. Nauta, R.E. Miller, Science 287, 293 (2000)
C.J. Burnham, S.S. Xantheas, M.A. Miller, B.E. Applegate, R.E. Miller, J. Chem. Phys. 117, 1109 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, Y., Moro, R. & Kresin, V. Changing the fragmentation pattern of molecules in helium nanodroplets by co-embedding with water . Eur. Phys. J. D 43, 109–112 (2007). https://doi.org/10.1140/epjd/e2007-00075-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjd/e2007-00075-y