Abstract.
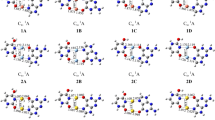
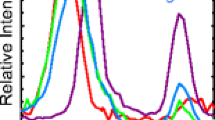
In recent work Gutowski et al. [Eur. Phys. J. D 20, 431 (2002)] reported photoelectron-spectroscopy and theoretical study of covalent anion of the uracil-glycine complex. In present work we use ab initio calculations to describe an anionic complex of uracil and glycine where the excess electron is localized in a diffuse state between the two monomers. In this system the uracil and glycine molecules are separated by about 4.5 Å and the dipoles of the two monomers point at the excess electron located in the middle of the complex. The calculated fragmentation energy of the anion into a dipole-bound uracil anion and a neutral glycine molecule is 1.7 kcal/mol.
Similar content being viewed by others
References
I. Galetich, S.G. Stepanian, V. Shelkovsky, M. Kosevich, L. Adamowicz, Mol. Phys. 100, 3649 (2002)
I. Galetich, S.G. Stepanian, V. Shelkovsky, M. Kosevich, Yu.P. Blagoi, L. Adamowicz, J. Phys. Chem. A 104, 8965 (2000)
I. Galetich, S.G. Stepanian, V. Shelkovsky, M. Kosevich, Yu.P. Blagoi, L. Adamowicz, J. Phys. Chem. B 103, 11211 (1999)
I. Galetich, M. Kosevich, V. Shelkovsky, S.G. Stepanian, Yu.B. Blagoi, L. Adamowicz, J. Mol. Struct. 478, 155 (1999)
J.E. Anderson, M. Ptashne, S.C. Harrison, Nature 326, 846 (1987)
T.I. Smolyaninova, V.I. Bruskov, Ye.V. Kashparova, Molec. Biol. (Russ.) 19, 992 (1985)
C. Helene, G. Lancelot, Prog. Biophys. Molec. Biol. 39, 1 (1982)
K.T. O’Neil, R.H. Hoess, W.F. DeGrado, Science 249, 774 (1990)
S.Y. Wodak, M.Y. Lin, H.W. Wyckoff, J. Molec. Biol. 116, 855 (1977)
L. Fairall, J.W.R. Schwabe, L. Chapman, J.T. Finch, D. Rhodes, Nature 366, 483 (1993)
R.S. Hedge, S.R. Grossman, L.A. Lainins, P.B. Sigler, Nature 359, 505 (1992)
Y. Kim, J.H. Geiger, S. Hahn, P.B. Sigler, Nature 365, 512 (1993)
J.L. Kim, D.B. Nikolov, S.K. Burley, Nature 365, 520 (1993)
R. Arni, U. Heinemann, R. Tokuoka, W. Saenger, J. Biol. Chem. 263, 15358 (1988)
K. Aflatooni, G.A. Gallup, P.D. Burrow, J. Phys. Chem. A 102, 6205 (1998)
V. Periquet, A. Moreau, S. Carles, J.P. Schermann, C.J. Desfrançois, Electron Spectrosc. Relat. Phenom. 106, 141 (2000)
C.J. Desfrançois, V. Periquet, Y. Bouteiller, J.P. Schermann, J. Phys. Chem. A 102, 1274 (1998)
S.D. Wetmore, R.J. Boyd, L.A. Eriksson, Chem. Phys. Lett. 322, 129 (2000)
S.S. Wesolowski, M.L. Leininger, P.N. Pentchew, H.F. Schaefer III, J. Am. Chem. Soc. 123, 4023 (2001)
M.D. Sevilla, B. Besler, A.O. Colson, J. Phys. Chem. 99, 1060 (1995)
G.H. Roehrig, N.A. Oyler, L. Adamowicz, J. Phys. Chem. 99, 14285 (1995)
E.C.M. Chen, E.S. Chen, J. Phys. Chem. B 104, 7835 (2000)
X. Li, Z. Cai, M.D. Sevilla, J. Phys. Chem. B 105, 10115 (2001)
X. Li, Z. Cai, M.D. Sevilla, J. Phys. Chem. 106, 1596 (2002)
N.A. Oyler, L. Adamowicz, J. Phys. Chem. 97, 11122 (1993)
A.O. Colson, B. Besler, M.D. Sevilla, J. Phys. Chem. 96, 9787 (1992)
J. Smets, A.F. Jalbout, L. Adamowicz, Chem. Phys. Lett. 342, 342 (2001)
N.J. Saettel, O. Wiest, J. Am. Chem. Soc. 123, 2693 (2001)
X. Li, Z. Cai, M.D. Sevilla, J. Phys. Chem. B 105, 10115 (2001)
I. Al-Jihad, J. Smets, L. Adamowicz, J. Phys. Chem. A 104, 2994 (2000)
N.A. Richardson, S.S. Wesolowski, H.F. Schaefer III, J. Phys. Chem. 107, 848 (2003)
M. Gutowski, I. Dabrowska, J. Rak, S. Xu, J.M. Nilles, D. Radisic, K.H. Bowen Jr, Eur. Phys. J. D 20, 431 (2002)
C. Desfrançois, H. Abdul-Carime, J.P. Schermann, J. Chem. Phys. 104, 7792 (1996)
J.H. Hendricks, S.A. Lyapustina, H.L. de Clercq, J.T. Snodgrass, K.H. Bowen, J. Chem. Phys. 104, 7788 (1996)
J.H. Hendricks, S.A. Lyapustina, H.L. de Clercq, K.H. Bowen, J. Chem. Phys. 108, 8 (1998)
M. Gutowski, C.S. Hall, L. Adamowicz, J.H. Hendricks, H.L. de Clercq, S.A. Lyapustina, J.M. Nilles, S.-J. Xu, K.H. Bowen, Phys. Rev. Lett. 88, 143003 (2002)
A.F. Jalbout, C.S. Hall, L. Adamowicz, Chem. Phys. Lett. 354, 128 (2002)
R.N. Compton, F.B. Dunning, P. Nordlander, Chem. Phys. Lett. 253, 8 (1996)
Kwang S. Kim, Ickjin Park, Sik Lee, K. Cho, Jin Yong Lee, Jongseob Kim, J.D. Joannopoulos, Phys. Rev. Lett. 76, 956 (1996)
M.J. Frisch , Gaussian 98, Revision A.7, Gaussian, Inc., Pittsburgh PA, 1998
Author information
Authors and Affiliations
Corresponding author
Additional information
Received: 18 April 2003, Published online: 22 July 2003
PACS:
31.15.Ar Ab initio calculations - 32.10.Hq Ionization potentials, electron affinities - 36.40.Wa Charged clusters
Rights and permissions
About this article
Cite this article
Jalbout, A.F., Pichugin, K.Y. & Adamowicz, L. An excess electron connects uracil to glycine. Eur. Phys. J. D 26, 197–200 (2003). https://doi.org/10.1140/epjd/e2003-00224-4
Issue Date:
DOI: https://doi.org/10.1140/epjd/e2003-00224-4