Abstract
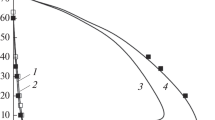
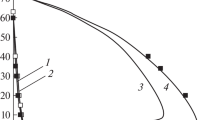
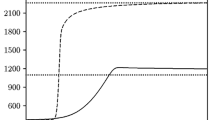
The contributions from various elementary reactions to the overall heat evolution rate in the propagation of hydrogen-air flames at atmospheric pressure have been determined. The study has been carried out by solving the system of kinetic equations and a heat balance equation, involving component concentration and temperature data available from the literature. The key role in heat evolution and in the propagation of the steady-state combustion wave is played by the set of reactions that lead to reaction chain branching. The contribution from the hydrogen atom recombination reaction to the overall heat evolution does not exceed 7% even for rich mixtures and cannot ensure flame propagation without contributions from other exothermic reactions.
Similar content being viewed by others
References
Semenov, N.N., O nekotorykh problemakh khimicheskoi kinetiki i reaktsionnoi sposobnosti (Topics in Chemical Kinetics and Reactivity), Moscow: Akad. Nauk SSSR, 1958.
Baldwin, R.R. and Simons, R.F., Trans. Faraday Soc., 1957, vol. 53, p. 955.
Karmilova, L.V., Nalbandyan, A.B., and Semenov, N.N., Zh. Fiz. Khim., 1958, vol. 32, no. 6, p. 1193.
Voevodskii, V.V., Fizika i khimiya elementarnykh khimicheskikh protsessov (Physics and Chemistry of Elementary Chemical Porcesses), Moscow: Nauka, 1969.
Azatyan, V.V. and Semenov, N.N., Kinet. Katal., 1972, vol. 13, no. 1, p. 17.
Lewis, B. and von Elbe, G., Combustion, Flames and Explosions of Gases, New York: Academic, 1987..
Azatyan, V.V., Nalbandyan, A.B., and Tsui Men-Yuan’, Dokl. Akad. Nauk SSSR, 1962, vol. 147, no. 2, p. 361.
Azatyan, V.V., Romanovich, L.B., and Sysoeva, S.G., Fiz. Goreniya Vzryva, 1967, vol. 2, no. 1, p. 77.
Azatyan, V.V., Gontkovskaya, V.T., and Merzhanov, A.G., Fiz. Goreniya Vzryva, 1973, vol. 9, no. 2, p. 163.
Azatyan, V.V., Andreeva, N.V., and El’natanov, A.I., Khim. Fiz., 1988, vol. 7, no. 8, p. 821.
Azatyan, V.V., Andrianova, Z.S., and Ivanova, A.N., Khim. Fiz., 1998, vol. 17, no. 8, p. 117.
Azatyan, V.V., Andrianova, Z.S., and Ivanova, A.N., Kinet. Catal., 2010, vol. 51, no. 3, p. 337.
Azatyan, V.V., Gaganidze, K.I., Kolesnikov, S.A., and Trubnikov, G.R., Kinet. Katal., 1982, vol. 23, no. 1, p. 244.
Baulch, D.L., Bowman, C.T., Cobos, C.J., Bauman, C.T., Just, Th., Troe, J., Walker, R.W., and Warnatz, J., J. Phys. Chem. Ref. Data, 2005, vol. 34, no. 3, p. 757.
Termodinamicheskie svoistva individual’nykh veshchestv (Thermodynamic Properties of Individual Compounds), Glushko, V.P., Ed., Moscow: Akad. Nauk SSSR, 1962.
Energii razryva khimicheskikh svyazei, potentsialy ionizatsii i srodstvo k elektronu (Bond Dissociation Energies, Ionization Potentials, and Electron Affinity), Kondrat’ev, V.I., Ed., Moscow: Nauka, 1974.
Peters, N. and Rogg, B., Reduced Kinetic Mechanisms for Applications in Combustion Systems, Berlin: Springer, 1992.
Petrova, L.D., Azatyan, V.V., Baratov, A.N., Vogman, L.P., and Makeev, V.I., in Gorenie i vzryv (Combustion and Explosion), Moscow: Nauka, 1977, p. 526.
Azatyan, V.V., Kinet. Catal., 1996, vol. 37, no. 4, p. 461.
Azatyan, V.V., Borisov, A.A., Merzhanov, A.G., and Kalachev, V.I., Fiz. Goreniya Vzryva, 2005, vol. 41, no. 1, p. 3.
Azatyan, V.V., Andrianova, Z.S., and Ivanova, A.N., Russ. J. Phys. Chem., 2006, vol. 80, no. 7, p. 1044.
Azatyan, V.V., Andrianova, Z.S., and Ivanova, A.N., Kinet. Catal., 2010, vol. 51, no. 4, p. 461.
Korobeinichev, O.P., Shmakov, A.G., Rybitskaya, I.V., Bolysheva, E.A., Chernov, A.A., Knyaz’kov, D.A., and Konnov, A.A., Kinet. Catal., 2009, vol. 50, no. 2, p. 156.
Azatyan, V.V. and Shavard, A.A., Kinet. Katal., 1977, vol. 18, no. 3, p. 596.
Zel’dovich, Ya.B., Kinet. Katal., 1961, vol. 2, no. 2, p. 305.
Zel’dovich, Ya.B., Barenblat, G.I., Librovich, V.B., and Makhviladze, G.M., Matematicheskaya teoriya goreniya i vzryva (Mathematical Theory of Combustion and Explosion), Moscow: Nauka, 1980.
Rubtsov, N.M., Seplyarskii, B.S., Chernish, V.I., and Tsvetkov, G.I., Mendeleev Commun., 2008, vol. 18, no. 1, p. 105.
Rubtsov, N.M., Seplyarskii, B.S., Chernysh, V.I., and Tsvetkov, G.I., Theor. Found. Chem. Eng., 2008, vol. 42, no. 6, p. 882.
Warnatz, J., Maas, U., and Dibble, R.W., Combustion: Physical and Chemical Fundamentals, Modeling and Simulations, Experiments, Pollutant Formation, Heidelberg: Springer, 2001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Azatyan, Z.S. Andrianova, A.A. Borisov, A.N. Ivanova, 2012, published in Kinetika i Kataliz, 2012, Vol. 53, No. 6, pp. 683–689.
Rights and permissions
About this article
Cite this article
Azatyan, V.V., Andrianova, Z.S., Borisov, A.A. et al. Main reactions determining heat evolution in hydrogen-oxygen combustion. Kinet Catal 53, 641–647 (2012). https://doi.org/10.1134/S002315841206002X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002315841206002X