Abstract
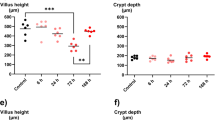
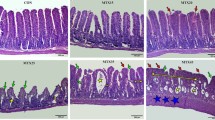
Chemotherapy-induced mucositis is an important dose-limiting and costly side effect for which there is no definitive prophylaxis or treatment. This is due in part to the lack of understanding of its pathophysiology and impact on intestinal function. The objectives of this study were to investigate the small intestine barrier function and electrolyte and water transport in an experimental model of methotrexate-induced mucositis, and to correlate these alterations with histological damage. Wistar rats were treated with methotrexate (1.5–3.5 mg/kg) for 3 days to induce mucositis. Intestinal permeability was measured by the urinary excretion rate of lactulose and mannitol following administration by gavage. Intestinal perfusion was performed in vivo for evaluation of water and electrolyte transports. Methotrexate-treated rats lost a significant amount of weight and presented a marked reduction in food intake. Methotrexate induced significant and dose-dependent villous atrophy and elongation of crypts in duodenum, jejunum, and ileum. Methotrexate also induced an increase in sodium and potassium secretion and an important reduction of the mucosa absorptive surface area, shown by the decrease in the mannitol excretion ratio. In conclusion, methotrexate caused major changes in small bowel function by disrupting intestinal permeability and inducing electrolyte secretion in parallel with substantial histological damage.
Similar content being viewed by others
REFERENCES
Sonis ST: Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 34:39–43, 1998
Pico JL, Avila-Garavito A, Naccache P: Mucositis: Its occurrence, consequences, and treatment in the oncology setting. Oncologist 3:446–451, 1998
Parrilli G, Laffaioli RV, Martorano M, Cuomo R, Tafuto S, Zampino MG, Budillon G, Bianco AR: Effects of anthracycline therapy on intestinal absorption in patients with advanced breast cancer. Cancer Res 49:3689–3691, 1989
Pledger JV, Pearson AD, CraftAW, LakerMF, Eastham EJ: Intestinal permeability during chemotherapy for childhood tumours. Eur J Pediatr 147:123–127, 1988
Trier JS: Morphologic alterations induced by methotrexate in the mucosa of human proximal intestine. Serial observations by light microscopy. Gastroenterology 42:295–305, 1962
Altmann GG: Changes in the mucosa of the small intestine following methotrexate administration or abdominal X-irradiation. Am J Anat 140:263–279, 1974
Potten CS: Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am J Physiol 273:G253–G257, 1997
Pritchard DM, Potten CS, Hickman JA: The relationships between p53-dependent apoptosis, inhibition of proliferation, and 5-fluorouracil-induced histopathology in murine intestinal epithelia. Cancer Res 58:5453–5465, 1998
Gibson RJ, Keefe DM, Clarke JM, Regester GO, Thompson FM, Goland GJ, Edwards BG, Cummins AG: The effect of keratinocyte growth factor on tumour growth and small intestinal mucositis after chemotherapy in the rat with breast cancer. Cancer Chemother Pharmacol 50:53–58, 2002
Donaldson SS, Lenon RA: Alterations of nutritional status: Impact of chemotherapy and radiation therapy. Cancer 43:2036–2052, 1979
Keefe DM, Cummins AG, Dale BM, Kotasek D, Robb TA, Sage RE: Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci (Colch) 92:385–389, 1997
Takeuchi H, Kosakai Y, Tsurui K, Hasegawa K, Horie T, Awazu S: Change in small intestinal brush border membranes of rats following methotrexate administration. Pharmacol Toxicol 65:269–273, 1989
Sundstrom GM, Wahlin A, Nordin-Andersson I, Suhr OB: Intestinal permeability in patients with acute myeloid leukemia. Eur J Haematol 61:250–254, 1998
Nakamaru M, Masubuchi Y, Narimatsu S, Awazu S, Horie T: Evaluation of damaged small intestine of mouse following methotrexate administration. Cancer Chemother Pharmacol 41:98–102, 1998
Hejna M, Brodowicz T, Zielinski CC: Local use of GM-CSF for severe mucositis. Eur J Cancer 35:S14–S17, 1999
Symonds RP: Treatment-induced mucositis: an old problem with new remedies. Br J Cancer 77:1689–1695, 1998
Taminiau JA, Gall DG, Hamilton JR: Response of the rat small-intestine epithelium to methotrexate. Gut 21:486–492, 1980
Erdman SH, Hart MH, Park JH, Vanderhoof JA: The intestinal absorption of 3-O-methyl-D-glucose in methotrexate-treated rats: An in vivo study of small bowel function. J Pediatr Gastroenterol Nutr 13:360–366, 1991
Naruhashi K, Nadai M, Nakao M, Suzuki N, Nabeshima T, Hasegawa T: Changes in absorptive function of rat intestine injured by methotrexate. Clin Exp Pharmacol Physiol 27:980–986, 2000
Papaconstantinou HT, Xie C, Zhang W, Ansari NH, Hellmich MR, Townsend CM Jr, Ko TC: The role of caspases in methotrexate-induced gastrointestinal toxicity. Surgery 130:859–865, 2001
Smith FP, Kisner DL, Widerlite L, Schein PS: Chemotherapeutic alteration of small intestinal morphology and function: a progress report. J Clin Gastroenterol 1:203–207, 1979
Vanderhoof JA, Park JH, Mohammadpour H, Blackwood D: Effects of dietary lipids on recovery from mucosal injury. Gastroenterol 98:1226–1231, 1990
Schedl HP, Clifton JA: Small intestinal absorption of steroids. Gastroenterology 41:491–499, 1961
Lima AA, Silva TM, Gifoni AM, Barrett LJ, McAuliffe IT, Bao Y, Fox JW, Fedorko DP, Guerrant RL: Mucosal injury and disruption of intestinal barrier function in HIV-infected individuals with and without diarrhea and cryptosporidiosis in northeast Brazil. Am J Gastroenterol 92:1861–1866, 1997
Pearson ADJ, Eastham EJ, LakerMF, CraftAW, Nelson R: Intestinal permeability in children with Crohn's disease and coeliac disease. Br Med J 285:20–21, 1982
Deitch EA: Intestinal permeability is increased in burn patients shortly after injury. Surgery 107:411–416, 1990
Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H: Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 341:1437–1439, 1993
Kohout P, Cerman J, Bratova M, Zadak Z: Small bowel permeability in patients with cytostatic therapy. Nutrition 15:546–549, 1999
Case DC, Jr., Gams R, Ervin TJ, Boyd MA, Oldham FB: Phase I-II trial of high-dose epirubicin in patients with lymphoma. Cancer Res 47:6393–6396, 1987
Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA: The pharmacology and clinical use of methotrexate. N Engl J Med 309:1094–1104, 1983
Capel ID, Pinnock MH, Williams DC: An in vitro assessment of the effect of cytotoxic drugs upon the intestinal absorption of nutrients in rats. Eur J Cancer 15:127–131, 1979
Cunningham D, Morgan RJ, Mills PR, Nelson LM, Toner PG, Soukop M, McArdle CS, Russell Rl: Functional and structural changes of the human proximal small intestine after cytotoxic therapy. J Clin Pathol 38:265–270, 1985
Altmann GG, Enesco M: Cell number as a measure of distribution and renewal of epithelial cells in the small intestine of growing and adult rats. Am J Anat 121:319–336, 1967
Loehry CA, Croft DN, Singh AK, Creamer B: Cell turnover in the rat small intestinal mucosa: An appraisal of cell loss. II. Cell loss in rats with an abnormal mucosa. Gut 10:16–18, 1969
Fox AD, Kripke SA, De Paula J, Berman JM, Settle RG, Rombeau JL: Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. J Parenter Enteral Nutr 12:325–331, 1988
Cobden I, Rothwell J, Axon AT: Passive permeability in experimental intestinal damage in rats. Clin Sci (Lond) 60:115–118, 1981
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carneiro-Filho, B.A., Lima, I.P.F., Araujo, D.H. et al. Intestinal Barrier Function and Secretion in Methotrexate-Induced Rat Intestinal Mucositis. Dig Dis Sci 49, 65–72 (2004). https://doi.org/10.1023/B:DDAS.0000011604.45531.2c
Issue Date:
DOI: https://doi.org/10.1023/B:DDAS.0000011604.45531.2c