Abstract
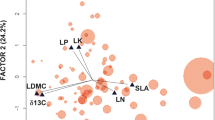
Litter decomposition, a major determinant of ecosystem functioning, is strongly influenced by the litter quality of different species. We aimed at (1) relating interspecific variation in leaf litter decomposition rate to the functional types different species belong to; and (2) understanding the chemical and/or physical basis for such variation and its robustness to environmental factors. We selected 52 Angiosperms from a climatic gradient in central-western Argentina, representing the widest range of functional types and habitats published so far. Ten litter samples of each species were simultaneously buried for 9 weeks during the 1996 summer in an experimental decomposition bed. Decomposition rate was defined as the percentage of dry mass loss after incubation. Chemical litter quality was measured as carbon (C) content, nitrogen (N) content, and C-to-N ratio. Since tensile strength of litter and living leaves were strongly correlated, the latter was chosen as an indicator of physical litter quality. A subset of 15 species representing different functional types was also incubated in England for 15 weeks, following a similar experimental procedure. Litter C-to-N and leaf tensile strength of the leaves showed the strongest negative associations with decomposition rate, both at the species and at the functional-type level. Decomposition rates of the same species in Argentina and in England were strongly correlated. This reinforces previous evidence that species rankings in terms of litter decomposition rates are robust to methodological and environmental factors. This paper has shown new evidence of plant control over the turnover of organic matter through litter quality, and confirms, over a broad spectrum of functional types, general models of resource allocation. The strong correlations between leaf tensile strength – a trait that is easy and quick to measure in a large number of species – decomposition rate, and C-to-N ratio indicate that leaf tensile strength can be useful in linking plant quality to decomposition patterns at the ecosystem level.
Similar content being viewed by others
References
Aerts R 1997 Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79, 439–449.
Aerts R and De Caluwe H 1997 Nutritional and plant-mediated controls on leaf litter decomposition of Carex species. Ecology 78, 244–260.
Ågren G I and Bosatta E 1996 Quality: a bridge between theory and experiment in soil organic matter studies. Oikos 76, 522–528.
Anderson J M 1991 The effects of climate change on decomposition processes in grassland and coniferous forests. Ecol. Appl. 1, 326–347.
Berendse F 1994 Litter decomposability - a neglected component of plant fitness. J. Ecol. 82, 187–190.
Burrows C J 1990 Processes of vegetation change. Unwin Hyman, London.
Cabido M 1985 Las comunidades vegetales de la Pampa de Achala, Sierras de Córdoba, Argentina. Documents Phytosociologiques 9, 431–443.
Cabido M, Acosta A and Díaz S 1989 Estudios fitosociológicos en pastizales de las Sierras de Córdoba, Argentina. Las comunidades de la Pampa de San Luis. Phytocoenologia 17, 569–592.
Cabido M, Acosta A and Díaz S 1990 The vascular flora and vegetation of granitic outcrops in the upper Córdoba mountains (Argentina). Phytocoenologia 19, 267–281.
Cabido M, Manzur A, Carranza M L and González C 1993 La vegetación y el medio físico del Chaco Árido en la Provincia de Córdoba, Argentina Central. Phytocoenologia 24, 423–460.
Cadisch G and Giller K E 1997 Driven by Nature: Plant Litter Quality and Decomposition. CAB International-University Press, Cambridge.
Capitanelli R G 1979 Bosquejo geomorfológico de la Provincia de Córdoba. Revista del IGM 5, 66–70.
Cepeda-Pizarro J G and Whitford W G 1989 Decomposition patterns of surface leaf litter of six species along a Chihuahuan Desert watershed. Am. Midd. Nat. 123, 319–330.
Chapin F S III 1980 The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 2, 233–260.
Chapin F S III, Autumn K and Pugnaire F 1993. Evolution of suites of traits in response to environmental stress. Am. Nat. 142 (Suppl.), 78–92.
Chapin F S III, Walker B H, Hobbs R J, Hooper D U, Lawton J H, Sala O E and Tilman D 1997 Biotic control over the functioning of ecosystems. Science 277, 500–504.
Choong M F 1996 What makes a leaf tough and how this affects the pattern of Castanopsis fissa leaf consumption by caterpillars. Func. Ecol. 10, 668–674.
Choong M F, Lucas P W, Ong J S Y, Pereira B, Tan H T W and Turner I M 1992 Leaf fracture toughness and sclerophylly: their correlations and ecological implications. New Phytol. 121, 597–610.
Coley P D 1980 Effects of leaf age and plant life history patterns on herbivory. Nature 283, 545–546.
Coley P D, Bryant J P and Chapin F S III 1985 Resource availability and plant antiherbivore defence. Science 230, 895–899.
Cornelissen J H C 1996 An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J. Ecol. 84, 573–582.
Cornelissen J H C and Thompson K 1997 Functional leaf attributes predict litter decomposition rate in herbaceous plants. New Phytol. 135, 109–114.
Cornelissen J H C, Pérez-Harguindeguy N, Díaz S, Grime J P, Marzano B, Cabido M, Vendramini F and Cerabolini B 1999 Leaf structure and defence control litter decomposition rate across species and life forms in regional floras of two continents. New Phytol. 143, 191–200.
Cotrufo M F, Ineson P and Rowland A P 1994 Decomposition of tree leaf litter grown under elevated CO2: Effect on litter quality. Plant Soil 163, 121–130.
Côuteaux M M, Bottner P and Berg B 1995 Litter decomposition, climate and litter quality. Trends Ecol. Evol. 10, 63–66.
Díaz S and Cabido M 1997 Plant functional types and ecosystem function in response to global change: a multiscale approach. J. Veg. Sci. 8, 463–474.
Gallardo A and Merino J 1993 Leaf decomposition in two Mediterranean ecosystems of Southwest Spain - influence of substrate quality. Ecology 74, 152–161.
Grime J P 1997 Biodiversity and ecosystem function: the debate deepens. Science 277, 1260–1261.
Grime J P 1979 Plant Strategies and Vegetation Processes. J Wiley and Sons, Chichester.
Grime J P, Cornelissen J H C, Thompson K and Hodgson J G 1996 Evidence of a causal connection between anti-herbivore defence and the decomposition rate of leaves. Oikos 77, 489–494.
Heal O W and Ineson P 1984 Carbon and energy flow in terrestrial ecosystems: relevance to microflora. In Current Perspectives in Microbial Ecology. Eds M J Klug and C A Reddy. pp 394–404. Amer. Soc. Microbiol., Washington.
Hendry G A F and Grime J P 1993 Methods in comparative plant ecology: a laboratory manual. Chapman and Hall, London.
Herms D A and Mattson W J 1992 The dilemma of plants: to grow or defend. Quart. Rev. Biol. 67, 283–335.
Hobbie S E 1992 Effects of plant species on nutrient cycling. Trends Ecol. Evol. 7, 336–339.
Hobbie S E 1996 Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol. Monogr. 66, 503–522.
Hollander M and Wolfe D A 1972 Non-parametric Statistical Methods. John Wiley and Sons, New York.
Hooper D U and Vitousek P M 1997 The effects of plant composition and diversity on ecosystem processes. Science 277, 1302–1305.
Howard P J A and Howard D M 1974 Microbial decomposition of tree and shrub leaf litter. 1. Weight loss and chemical composition of decomposing litter. Oikos 25, 341–352.
Huntley B, Cramer W, Morgan A V, Prentice H C and Allen J R M 1997 Past and Future Rapid Environmental Changes: the Spatial and Evolutionary Responses of Terrestrial Biota. NATO ASI Series 1. Springer, Berlin.
Iritani W M and Arnold C Y 1960 Nitrogen release of vegetable crop residues during incubation as related to their chemical composition. Soil Science 89, 74–82.
Lavelle P, Blanchart E, Martin A, Martin S, Spain A V, Toutain F, Barois I and Schaefer R 1993 A hierarchical model for decomposition in terrestrial ecosystems: applications to soils of the humid tropics. Biotropica 25, 130–150.
McClaugherty C A, Pastor J, Aber J D and Melillo J M 1985 Forest litter decomposition in relation to soil nitrogen dynamics and litter quality. Ecology 66, 266–275.
Meentemeyer V 1978 Macroclimate and lignin control over litter decomposition rates. Ecology 59, 465–472.
Melillo J M, Aber J D and Muratore J F 1982 Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63, 571–584.
Mtambanengwe F and Kirchmann H 1995 Litter from a tropical savanna woodland (MIOMBO): chemical composition and C and N mineralization. Soil Biol. Biochem. 27, 1639–1651.
Murphy K L, Klopatek J M and Klopatek C C 1998 The effect of litter quality and climate on decomposition along an elevational gradient. Ecol. Appl. 8, 1061–1071.
Nicolai V 1988 Phenolic and mineral content of leaves inflluences decomposition in European forest ecosystems. Oecologia 75, 575–579.
Norby R J and Cotrufo M F 1998 A question of litter quality. Nature 396, 17–18.
Norusis M J 1992 SPSS for Windows base system user's guide, Release 5.0. SPSS Inc., Chicago.
Palm C A and Rowland A P 1997 A minimum dataset for characterization of plant quality for decomposition. In Driven by Nature: Plant Litter Quality and Decomposition. Eds G Cadisch and K E Giller. pp 379–392. CAB International-University Press, Cambridge.
Pérez-Harguindeguy N, Díaz S, Cornelissen J H C and Cabido M 1997 Comparación experimental de la tasa de descomposición foliar potencial de especies vegetales del centro-oeste de Argentina. Ecología Austral 7, 87–94.
Seastedt T R, Crossley D A Jr, Meentemeyer V and Waide J B 1983 A two-year study of leaf litter decomposition as related to microclimatic factors and microarthropods abundance in the southern Appalachians. Holarctic Ecology 6, 11–16.
Schaefer D, Steinberger Y and Whitford W G 1985 The failure of nitrogen and lignin decomposition in a North American Desert ecosystem. Oecologia 65, 383–386.
Shlelinger W H 1977. Carbon balance in terrestrial detritus. Ann. Rev. Ecol. Syst. 8, 51–81.
Swift M J, Heal O W and Anderson J M 1979 Decomposition in Terrestrial Ecosystems. Studies in Ecology 5. Blackwell, Oxford.
Taylor B R, Parkinson D, Parsons W J F 1989 Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70, 97–104.
Tilman D, Knops J, Wedin J D, Reich P B, Ritchie M and Siemann E 1997 The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302.
van der Putten W H 1997 Plant-soil feedback as a selective force. Trends Ecol. Evol. 12, 169–170.
Vitousek P M, Mooney H A, Lubchenko J and Melillo J M 1997 Human domination of Earth's ecosystem. Science 277, 494–499.
Wardle D A, Bonner K I and Nicholson K S 1997 Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 79, 247–258.
Wardle D A, Barker G M, Bonner K I and Nicholson K S 1998 Can comparative approaches based on plant ecophysiological traits predict the nature of biotic interactions and individual plant species effects in ecosystems? J. Ecol. 86, 405–420.
Wright W and Illius A W 1995 A comparative study of the fracture properties of five grasses. Func. Ecol. 9, 269–278.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pérez-Harguindeguy, N., Díaz, S., Cornelissen, J.H.C. et al. Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant and Soil 218, 21–30 (2000). https://doi.org/10.1023/A:1014981715532
Issue Date:
DOI: https://doi.org/10.1023/A:1014981715532