Abstract
Background
Dapagliflozin’s antihyperglycemic effects are mediated by inhibition of renal sodium-glucose cotransporter-2; therefore, renal safety of dapagliflozin was assessed.
Methods
Twelve double-blind, placebo-controlled, randomized clinical trials were analyzed up to 24 weeks (N = 4545). Six of the 12 studies included long-term data for up to 102 weeks (N = 3036). Patients with type 2 diabetes with normal or mildly impaired renal function [estimated glomerular filtration rate (eGFR) 60 to <90 mL/min/1.73 m2] were treated with dapagliflozin (2.5, 5, or 10 mg/day) or placebo. Renal adverse events (AEs) were assessed.
Results
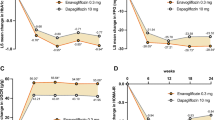
Mean eGFR showed small transient reductions with dapagliflozin at week 1, but returned to near baseline values by week 24 and remained stable to week 102. Mean eGFR changes were not very different for dapagliflozin 2.5, 5 and 10 mg versus placebo at 102 weeks: −0.74, 2.52 and 1.38 versus 1.31 mL/min/1.73 m2, respectively. Renal AEs were similar in frequency to placebo through 24 weeks (1.4, 1.3, 0.9, and 0.9 %, respectively) and 102 weeks (2.4, 1.8, 1.9 and 1.7 %, respectively). Few were serious (0.2, 0.1, 0 and 0.3 %, respectively, over 102 weeks). The most common renal event was serum creatinine increase. In sub-group analyses in patients ≥65 years of age or those with moderate renal impairment (eGFR 30 to <60 mL/min/1.73 m2), renal AEs occurred more frequently with dapagliflozin than placebo. No events of acute tubular necrosis were reported.
Conclusion
In patients with normal or mildly impaired renal function, dapagliflozin is not associated with increased risk of acute renal toxicity or deterioration of renal function. All trials included in this analysis are registered at ClinicalTrials.gov: NCT00263276, NCT00972244, NCT00528372, NCT00736879, NCT00528879, NCT00855166, NCT00357370, NCT00680745, NCT00683878, NCT00673231, NCT00643851, NCT00859898.

Similar content being viewed by others
References
Wood IS, Trayhurn P (2003) Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 891:3–9
Kanai Y, Lee WS, You G, Brown D, Hediger MA (1994) The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93:397–404
Komoroski B, Vachharajani N, Boulton D et al (2009) Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 85:520–526
Meng W, Ellsworth BA, Nirschl AA et al (2008) Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem 51:1145–1149
Han S, Hagan DL, Taylor JR et al (2008) Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 57:1723–1729
List JF, Whaley JM (2011) Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl 79:S20–S27
Mather A, Pollock C (2011) Glucose handling by the kidney. Kidney Int Suppl 79:S1–S6
Vallon V, Platt KA, Cunard R et al (2011) SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22:104–112
List JF, Woo V, Morales E, Tang W, Fiedorek FT (2009) Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32:650–657
Bailey CJ, Gross JL, Pieters A, Bastien A, List JF (2010) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375:2223–2233
Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF (2010) Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33:2217–2224
Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S (2011) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 13:928–938
Nauck MA, Del Prato S, Meier JJ et al (2011) Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 34:2015–2022
Rosenstock J, Vico M, Wei L, Salsali A, List JF (2012) Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 35:1473–1478
Wilding JP, Norwood P, T’Joen C, Bastien A, List JF, Fiedorek FT (2009) A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 32:1656–1662
Wilding JP, Woo V, Soler NG et al (2012) Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 156:405–415
Sharif A (2011) Current and emerging antiglycaemic pharmacological therapies: the renal perspective. Nephrol (Carlton) 16:468–475
Committee for Medicinal Products for Human Use European Public Assessment Report (EPAR) Canagliflozin European Medicines Agency 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002649/WC500156457.pdf
Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D et al (2014) Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 16:1016–1027
Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ et al (2014) Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2:369–384
Kaku K, Inoue S, Matsuoka O, Kiyosue A, Azuma H, Hayashi N et al (2013) Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 15:432–440
Bailey CJ, Iqbal N, T’joen C, List JF (2012) Dapagliflozin monotherapy in drug-naïve patients with diabetes: a randomized-controlled trial of low-dose range. Diabetes Obes Metab 14:951–959
Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM et al (2012) Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 97:1020–1031
Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF (2012) Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Prac 66:446–456
National Kidney Foundation (2002) Part 4. Definition and classification of stages of chronic kidney disease. Am J Kidney Dis 39:S46–S75
Vallon V, Richter K, Blantz RC, Thomson S, Osswald H (1999) Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10:2569–2576
Thomson SC, Rieg T, Miracle C et al (2012) Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302:R75–R83
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483
Kohan DE, Fioretto P, Tang W, List JF (2014) Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 85:962–971
Kohan DE, Fioretto P, List J, et al (2011) Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment. Presented at the American society of nephrology kidney week 2011 annual meeting; Philadelphia, PA, November 8–13, 2011; abstract #TH-PO524
Fukui M, Tanaka M, Shiraishi E et al (2008) Serum uric acid is associated with microalbuminuria and subclinical atherosclerosis in men with type 2 diabetes mellitus. Metabolism 57:625–629
Kodama S, Saito K, Yachi Y et al (2009) Association between serum uric acid and development of type 2 diabetes. Diabetes Care 32:1737–1742
Yale JF, Bakris G, Cariou B et al (2014) Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 16(10):1016–1027
Gembardt F, Bartaun C, Jarzebska N et al (2014) The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol 307(3):F317–F325
Vallon V, Gerasimova M, Rose MA et al (2014) SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306(2):F194–F204
Wanner C, Lachin JM, Fitchett DH, et al. (2015) Empagliflozin and clinical outcomes in patients with type 2 diabetes and chronic kidney disease. In: American Society of Nephrology, San Diego, CA, pp abstract# HI-OR01
Acknowledgments
The study team would like to acknowledge the patients for their participation and commitment during the studies. We also thank the investigators and contributors from each study site. The authors thank Dr. Bruce Leslie for his involvement with this analysis, Dr James List for his contribution to the analysis and initial manuscript development, and Jennifer Sugg for her contribution to statistical analysis. Professional medical writing and editorial assistance was provided by Carolyn Carroll, PhD, an employee of Bristol-Myers Squibb at the time of the development of this manuscript and Shelley Narula, MBBS, of inScience Communications, Springer Healthcare.
Funding
The conduct and analyses of these trials were supported by Bristol-Myers Squibb and AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DEK is a consultant for Bristol-Myers Squibb and AstraZeneca. PF is a consultant for Bristol-Myers Squibb and AstraZeneca, and participant in boards for Boehringer Ingelheim. KJ and SP are employees and shareholders of AstraZeneca. AP and LY are employees and shareholders of Bristol-Myers Squibb.
Ethical approval
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Informed consent
Informed consent was obtained from all participants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kohan, D.E., Fioretto, P., Johnsson, K. et al. The effect of dapagliflozin on renal function in patients with type 2 diabetes. J Nephrol 29, 391–400 (2016). https://doi.org/10.1007/s40620-016-0261-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-016-0261-1