Abstract
The norepinephrine prodrug droxidopa (NORTHERA™) is approved in the US for the treatment of orthostatic dizziness, lightheadedness, or the ‘feeling that you are about to black out’ in adults with symptomatic neurogenic orthostatic hypotension associated with primary autonomic failure (e.g. Parkinson’s disease, multiple system atrophy or pure autonomic failure), dopamine β-hydroxylase deficiency or nondiabetic autonomic neuropathy. This article reviews the clinical efficacy and tolerability of droxidopa in symptomatic neurogenic orthostatic hypotension, as well as summarizing its pharmacological properties. Oral droxidopa was effective in the shorter-term treatment of patients with symptomatic neurogenic orthostatic hypotension, with improvements seen in symptoms, the impact of symptoms on daily activities and standing systolic blood pressure. More data are needed to confirm the longer-term efficacy of droxidopa. Droxidopa was generally well tolerated, although patients should be monitored for supine hypertension.

Similar content being viewed by others
References
Freeman R, Landsberg L. The treatment of orthostatic hypotension with dihydroxyphenylserine. Clin Neuropharmacol. 1991;14(4):296–304.
Berger MJ, Kimpinski K. A practical guide to the treatment of neurogenic orthostatic hypotension. Can J Neurol Sci. 2014;41(2):156–63.
Schroeder C, Jordan J, Kaufmann H. Management of neurogenic orthostatic hypotension in patients with autonomic failure. Drugs. 2013;73(12):1267–79.
Lundbeck NA Ltd. NORTHERA™ (droxidopa) capsules, for oral use: US prescribing information. 2014. http://www.northera.com. Accessed 10 Dec 2014.
Goldstein DS. L-Dihydroxyphenylserine (L-DOPS): a norepinephrine prodrug. Cardiovasc Drug Rev. 2006;24(3–4):189–203.
Kaufmann H. The discovery of the pressor effect of DOPS and its blunting by decarboxylase inhibitors. J Neural Transm. 2006;70(Suppl):477–84.
Goldstein DS, Holmes C, Sewell LT, et al. Effects of carbidopa and entacapone on the metabolic fate of the norepinephrine prodrug L-DOPS. J Clin Pharmacol. 2011;51(1):66–74.
Kaufmann H. L-dihydroxyphenylserine (droxidopa): a new therapy for neurogenic orthostatic hypotension. The US experience. Clin Auton Res. 2008;18(Suppl 1):19–24.
Kaufmann H, Saadia D, Voustianiouk A, et al. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108(6):724–8.
Goldstein DS, Holmes C, Kaufmann H, et al. Clinical pharmacokinetics of the norepinephrine precursor L-threo-DOPS in primary chronic autonomic failure. Clin Auton Res. 2004;14(6):363–8.
Suzuki T, Higa S, Sakoda S, et al. Pharmacokinetic studies of oral L-threo-3,4-dihydroxyphenylserine in normal subjects and patients with familial amyloid polyneuropathy. Eur J Clin Pharmacol. 1982;23(5):463–8.
Suzuki T, Sakoda S, Ueji M, et al. Treatment of parkinsonism with L-threo-3,4-dihydroxyphenylserine: a pharmacokinetic study. Neurology. 1984;34(11):1446–50.
Kato T, Karai N, Katsuyama M, et al. Studies on the activity of L-threo-3,4-dihydroxyphenylserine (L-DOPS) as a catecholamine precursor in the brain: comparison with that of L-DOPA. Biochem Pharmacol. 1987;36(18):3051–7.
US Food and Drug Administration. Droxidopa: medical review. Application number: 203202Orig1s000. 2014. http://www.fda.gov. Accessed 10 Dec 2014.
LeWitt P, Gorny S. Analysis of efficacy and safety outcomes in patients treated with droxidopa in combination with other drug classes [abstract no. 1294]. Mov Disord. 2012;27(Suppl 1):S425–6.
Kaufmann H, Oribe E, Yahr MD. Differential effect of L-threo-3,4-dihydroxyphenylserine in pure autonomic failure and multiple system atrophy with autonomic failure. J Neural Transm. 1991;3(2):143–8.
Mathias CJ, Senard J-M, Braune S, et al. L-threo-dihydroxyphenylserine (L-threo-DOPS; droxidopa) in the management of neurogenic orthostatic hypotension: a multi-national, multi-center, dose-ranging study in multiple system atrophy and pure autonomic failure. Clin Auton Res. 2001;11:235–42.
Mathias CJ, Senard JM, Cortelli P. A double-blind, randomized, placebo-controlled study to determine the efficacy and safety of droxidopa in the treatment of orthostatic hypotension associated with multiple system atrophy or Parkinson’s disease [abstract]. Clin Auton Res. 2007;17:272.
Wikstrom L, Bjerle P, Boman K. L-threo-DOPS treatment of orthostatic hypotension in Swedish patients with familial amyloidotic polyneuropathy (TTR-met30). Amyloid J Protein Fold Disord. 1996;3:162–6.
Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83(4):328–35.
Biaggioni I, Freeman R, Mathias CJ, et al. Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa. Hypertension. 2014. doi:10.1161/HYPERTENSIONAHA.114.04035.
Hauser RA, Hewitt LA, Isaacson S. Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson’s disease (NOH306A). J Parkinsons Dis. 2014;4(1):57–65.
Hauser RA, Isaacson S, Lisk JP, et al. Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson’s disease (nOH306B). Mov Disord. 2014. doi:10.1002/mds.26086.
Isaacson S, Shill H, Vernino S, et al. Durability of effect with long-term, open-label droxidopa treatment in patients with symptomatic neurogenic orthostatic hypotension (NOH 303) [abstract no. 1291]. Mov Disord. 2012;27(Suppl 1):S424–5.
Mathias C, Low P, Freeman R, et al. Integrated efficacy analysis of droxidopa in 2 double-blind, placebo-controlled phase 3 studies in patients with neurogenic orthostatic hypotension [abstract no. 1296]. Mov Disord. 2012;27(Suppl 1):S426.
Isaacson S, Hauser R, Szakacs C, et al. Droxidopa treatment impact on orthostatic symptoms and standing systolic blood pressure in patients with Parkinson’s disease (PD) and symptomatic neurogenic orthostatic hypotension (NOH) [abstract]. Neurology. 2013;80(19):e203.
Hauser RA, Jerome LP, Schwieterman WD, et al. Impact of droxidopa treatment on falls and fall related injuries in patients with Parkinson’s disease and symptomatic neurogenic orthostatic hypotension (study 306) [abstract no. 481]. Mov Disord. 2013;28(Suppl 1):S171.
Shill H, Vernino S, Hutchman R, et al. A multicenter, open-label study to assess the long-term safety of droxidopa in patients with symptomatic neurogenic orthostatic hypotension (NOH 304) [abstract no. 1302]. Mov Disord. 2012;27(Suppl 1):S428.
Lamarre-Cliche M. Drug treatment of orthostatic hypotension because of autonomic failure or neurocardiogenic syncope. Am J Cardiovasc Drugs. 2002;2(1):23–35.
Upsher-Smith Laboratories Inc. Midodrine HCl tablets: US prescribing information. 2012. http://www.upsher-smith.com/. Accessed 10 Dec 2014.
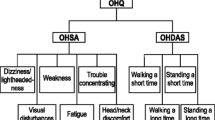
Kaufmann H, Malamut R, Norcliffe-Kaufmann L, et al. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22(2):79–90.
Disclosure
The preparation of this review was not supported by any external funding. Gillian Keating is a salaried employee of Adis/Springer. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: S.H. Fox, Movement Disorder Clinic, University of Toronto, Toronto Western Hospital, Toronto, ON, Canada; S. Grill, Parkinson’s and Movement Disorders Center of Maryland, Elkridge, MD, USA; P.A. Low, Mayo Clinic, Rochester, MN, USA.
Rights and permissions
About this article
Cite this article
Keating, G.M. Droxidopa: A Review of Its Use in Symptomatic Neurogenic Orthostatic Hypotension. Drugs 75, 197–206 (2015). https://doi.org/10.1007/s40265-014-0342-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0342-1