Abstract
Background
Crizotinib, ceritinib, and alectinib improved survival in patients with anaplastic lymphoma kinase (ALK) arrangement non-small-cell lung cancer (NSCLC); however, the long-term economic outcomes of using ceritinib and alectinib versus crizotinib are still unclear.
Objective
This analysis aimed to evaluate the cost effectiveness of ceritinib and alectinib versus crizotinib in the Chinese healthcare setting.
Methods
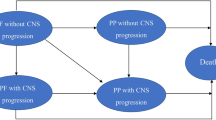
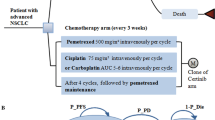
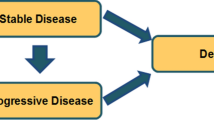
A Markov model was developed to project the economic and health outcomes for the treatment of advanced NSCLC with ceritinib, alectinib or crizotinib. A network meta-analysis was performed to calculate the hazard ratios of ceritinib and alectinib versus crizotinib by pooling published trials. Cost and utility values were obtained from the literature, and one-way and probabilistic sensitivity analyses were carried out to determine the robustness of the model outcomes. The primary outputs included total cost, life-years (LYs), quality-adjusted LYs (QALYs), and incremental cost-effectiveness ratio (ICER).
Results
Treatment with alectinib and ceritinib yielded an additional 1.00 and 1.09 QALYs and incremental costs of $62,232 and $15,165, resulting in an ICER of $62,231 and $13,905 per QALY compared with crizotinib, respectively. Parameters related to drug costs and progression-free survival were the main drivers of the model outcomes. From the probabilistic sensitivity analysis, ceritinib and alectinib had a 99.9% and 0% probability of being cost effective, respectively, at a willingness-to-pay threshold of US$28,410/QALY.
Conclusions
Our results indicate that compared with crizotinib and alectinib, ceritinib is a cost-effective option for treatment-naïve patients with ALK-positive advanced NSCLC.



Similar content being viewed by others
References
GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–922.
Christopoulos P, Budczies J, Kirchner M, Dietz S, Sultmann H, Thomas M, et al. Defining molecular risk in ALK(+) NSCLC. Oncotarget. 2019;10:3093–103.
Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. ALK inhibitors in the treatment of ALK positive NSCLC. Front Oncol. 2018;8:557.
Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, et al. NCCN Guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Cancer Netw. 2018;16:807–21.
Lyu C, Wu N. Management guidelines for primary lung cancer 2018: Chinese standards in clinical practice. Chin J Cancer Res. 2019;31:419–20.
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77.
Soria JC, Tan DS, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–29.
Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and markov decision-analytic modeling. Med Decis Mak. 2017;37:427–39.
Cho BC, Kim DW, Bearz A, Laurie SA, McKeage M, Borra G, et al. ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). J Thorac Oncol. 2017;12:1357–67.
Duruisseaux M, Besse B, Cadranel J, Perol M, Mennecier B, Bigay-Game L, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;8:21903–17.
Xiao J, Sun JF, Wang QQ, Qi X, Yao HY. Health economic evaluation reporting guideline and application status. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51:276–80.
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Thompson CJ, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al. Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation. Health Technol Assess. 2010;14:1–184.
Li J, Knoll S, Bocharova I, Tang W, Signorovitch J. Comparative efficacy of first-line ceritinib and crizotinib in advanced or metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer: an adjusted indirect comparison with external controls. Curr Med Res Opin. 2019;35:105–11.
Carlson JJ, Suh K, Orfanos P, Wong W. Cost effectiveness of alectinib vs. crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Pharmacoeconomics. 2018;36:495–504.
Zhou ZY, Mutebi A, Han S, Bensimon AG, Louise RM, Xie J, et al. Cost-effectiveness of ceritinib in previously untreated anaplastic lymphoma kinase-positive metastatic non-small cell lung cancer in the United States. J Med Econ. 2018;21:577–86.
Chinese Drug Price of Drug Centralized Bid Procurement; 2019. https://db.yaozh.com/yaopinzhongbiao. Accessed 30 May 2019.
Lu S, Zhang J, Ye M, Wang B, Wu B. Economic analysis of ALK testing and crizotinib therapy for advanced non-small-cell lung cancer. Pharmacogenomics. 2016;17:985–94.
Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13:e195–203.
Guan H, Sheng Y, Guo W, Han S, Shi L. Cost-effectiveness of alectinib for patients with untreated ALK-positive non-small cell lung cancer in China. Adv Ther. 2019;36:1114–25.
Li H, Liu GG, Wu J, Wu JH, Dong CH, Hu SL. Recent pricing negotiations on innovative medicines pilot in China: experiences, implications, and suggestions. Value Health Reg Issues. 2018;15:133–7.
Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Mak. 2013;33:607–17.
Spagnuolo A, Maione P, Gridelli C. Evolution in the treatment landscape of non-small cell lung cancer with ALK gene alterations: from the first- to third-generation of ALK inhibitors. Expert Opin Emerg Drugs. 2018;23:231–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hongchao Li, Lei Lai and Bin Wu have no conflicts of interest to declare.
Funding
No funding or sponsorship was received for this work.
Author Contributions
HL, LL and BW were involved in the design of the study, collected the data and performed the economic analysis. BW wrote the first draft of the manuscript, which was critically revised by LS.
Data Sharing
No additional data are available.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, H., Lai, L. & Wu, B. Cost Effectiveness of Ceritinib and Alectinib Versus Crizotinib in First-Line Anaplastic Lymphoma Kinase-Positive Advanced Non-small-cell Lung Cancer. Clin Drug Investig 40, 183–189 (2020). https://doi.org/10.1007/s40261-019-00880-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00880-8