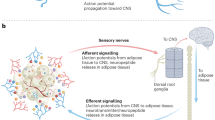
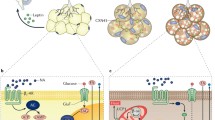
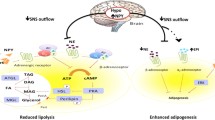
Abstract
Increasing energy expenditure is an appealing therapeutic target for the prevention and reversal of metabolic conditions such as obesity or type 2 diabetes. However, not enough research has investigated how to exploit pre-existing neural pathways, both in the central nervous system (CNS) and peripheral nervous system (PNS), in order to meet these needs. Here, we review several research areas in this field, including centrally acting pathways known to drive the activation of sympathetic nerves that can increase lipolysis and browning in white adipose tissue (WAT) or increase thermogenesis in brown adipose tissue (BAT), as well as other central and peripheral pathways able to increase energy expenditure of these tissues. In addition, we describe new work investigating the family of transient receptor potential (TRP) channels on metabolically important sensory nerves, as well as the role of the vagus nerve in regulating energy balance.

Similar content being viewed by others
Abbreviations
- Adrβ3:
-
Adrenergic receptor β3
- AgRP:
-
Agouti-related peptide
- ARC:
-
Arcuate nucleus
- BAT:
-
Brown adipose tissue
- BDNF:
-
Brain-derived neurotrophic factor
- BMP:
-
Bone morphogenetic protein
- CGRP:
-
Calcitonin gene-related peptide
- CNS:
-
Central nervous system
- DH:
-
Dorsal horn
- DIO:
-
Diet-induced obesity
- DRG:
-
Dorsal root ganglion
- FGF21:
-
Fibroblast growth factor 21
- HFD:
-
High-fat diet
- LHA:
-
Lateral hypothalamic area
- LXR:
-
Liver X receptor
- NG:
-
Nodose ganglion
- NGF:
-
Nerve growth factor
- NPY:
-
Neuropeptide Y
- NR:
-
Nuclear receptor
- OT:
-
Oxytocin
- OXTR:
-
Oxytocin receptor
- PAG:
-
Periaqueductal gray
- PGC-1α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PNS:
-
Peripheral nervous system
- POA:
-
Preoptic area
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- PRV152:
-
Pseudorabies virus 152
- PSNS:
-
Parasympathetic nervous system
- PVH:
-
Paraventricular hypothalamus
- SNS:
-
Sympathetic nervous system
- SP:
-
Substance P
- TG:
-
Trigeminal ganglion
- TH:
-
Tyrosine hydroxylase
- TRP:
-
Transient receptor potential
- TRPV:
-
Transient receptor potential vanilloid
- UCP1:
-
Uncoupling protein 1
- VMH:
-
Ventromedial hypothalamus
- VNS:
-
Vagus nerve stimulation
- WAT:
-
White adipose tissue
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
The World Health Organization. ISBN: 978 92 4 156485 4 http://www.who.int/nmh/publications/ncd-status-report-2014/en/. Retrieved: March 2016.
Ward ZJ, Long MW, Resch SC, Gortmaker SL, Cradock AL, Giles C, et al. Redrawing the US obesity landscape: bias-corrected estimates of state-specific adult obesity prevalence. PLoS One. 2016;11(3):e0150735.
Townsend KL, Tseng YH. Brown fat fuel utilization and thermogenesis. Trends Endocrinol Metab. 2014;25(4):168–77. Review.
Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63(12):4089–99.
Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276(6 Pt 2):R1569–78.
Vaughan CH, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am J Physiol Regul Integr Comp Physiol. 2012;302(9):R1049–58.
Ryu V, Garretson JT, Liu Y, Vaughan CH, Bartness TJ. Brown adipose tissue has sympathetic-sensory feedback circuits. J Neurosci. 2015;35(5):2181–90.
Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol. 2014;306(12):R886–900.
Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48(8):1655–72.
Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am J Physiol. 1995;268(3 Pt 2):R744–51.
Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, et al. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147(3):1140–7.
Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol. 1987;253(6 Pt 2):R942–4.
Burneo JG, Faught E, Knowlton R, Morawetz R, Kuzniecky R. Weight loss associated with vagus nerve stimulation. Neurology. 2002;59(3):463–4.
Abubakr A, Wambacq I. Long-term outcome of vagus nerve stimulation therapy in patients with refractory epilepsy. J Clin Neurosci. 2008;15(2):127–9.
Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes (Lond). 2007;31(11):1756–9.
Bugajski AJ, Gil K, Ziomber A, Zurowski D, Zaraska W, Thor PJ. Effect of long-term vagal stimulation on food intake and body weight during diet induced obesity in rats. J Physiol Pharmacol. 2007;58 Suppl 1:5–12.
Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34.
Banni S, Carta G, Murru E, Cordeddu L, Giordano E, Marrosu F, et al. Vagus nerve stimulation reduces body weight and fat mass in rats. PLoS One. 2012;7(9):e44813.
Saindon CS, Blecha F, Musch TI, Morgan DA, Fels RJ, Kenney MJ. Effect of cervical vagotomy on sympathetic nerve responses to peripheral interleukin-1beta. Auton Neurosci. 2001;87(2–3):243–8.
Balbo SL, Grassiolli S, Ribeiro RA, Bonfleur ML, Gravena C, Brito Mdo N, et al. Fat storage is partially dependent on vagal activity and insulin secretion of hypothalamic obese rat. Endocrine. 2007;31(2):142–8.
Mano-Otagiri A, Ohata H, Iwasaki-Sekino A, Nemoto T, Shibasaki T. Ghrelin suppresses noradrenaline release in the brown adipose tissue of rats. J Endocrinol. 2009;201(3):341–9.
Liu C, Bookout AL, Lee S, Sun K, Jia L, Lee C, et al. PPARγ in vagal neurons regulates high-fat diet induced thermogenesis. Cell Metab. 2014;19(4):722–30. Provides a novel mechanism by which vagus neurons help regulate energy balance.
Mansuy-Aubert V, Gautron L, Lee S, Bookout AL, Kusminski C, Sun K, et al. Loss of the liver X receptor LXRα/β in peripheral sensory neurons modifies energy expenditure. Elife. 2015;15:4.
Zheng J. Molecular mechanism of TRP channels. Compr Physiol. 2013;3(1):221–42.
Saito M, Yoneshiro T, Matsushita M. Food ingredients as anti-obesity agents. Trends Endocrinol Metab. 2015;26(11):585–7.
Planells-Cases R, Garcìa-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005;451(1):151–9.
Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100(7):1063–70.
Nathan JD, Patel AA, McVey DC, Thomas JE, Prpic V, Vigna SR, et al. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1322–8.
Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582(15):2257–62.
Blesch A, Tuszynski MH. GDNF gene delivery to injured adult CNS motor neurons promotes axonal growth, expression of the trophic neuropeptide CGRP, and cellular protection. J Comp Neurol. 2001;436(4):399–410.
Lee E, Jung DY, Kim JH, Patel PR, Hu X, Lee Y, et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015;29(8):3182–92.
Ohyama K, Nogusa Y, Suzuki K, Shinoda K, Kajimura S, Bannai M. A combination of exercise and capsinoid supplementation additively suppresses diet-induced obesity by increasing energy expenditure in mice. Am J Physiol Endocrinol Metab. 2015;308(4):E315–23.
Saito M. Capsaicin and related food ingredients reducing body fat through the activation of TRP and brown fat thermogenesis. Adv Food Nutr Res. 2015;76:1–28.
Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38(3–4):233–52.
Alawi KM, Aubdool AA, Liang L, Wilde E, Vepa A, Psefteli MP, et al. The sympathetic nervous system is controlled by transient receptor potential vanilloid 1 in the regulation of body temperature. FASEB J. 2015;29(10):4285–98. TRPV1 is involved in thermoregulation.
Luo Z, Ma L, Zhao Z, He H, Yang D, Feng X, et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice. Cell Res. 2012;22(3):551–64.
Cheung SY, Huang Y, Kwan HY, Chung HY, Yao X. Activation of transient receptor potential vanilloid 3 channel suppresses adipogenesis. Endocrinology. 2015;156(6):2074–86. Activation of TRPV3 channel inhibits adipogenesis in vitro and decreases visceral adiposity in vivo.
Sun W, Uchida K, Suzuki Y, Zhou Y, Kim M, Takayama Y, et al. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep. 2016;17(3):383–99. Although lack of TRPV2 channel increases brown adipocyte differentiation, it impairs the thermogenic function of the tissue.
Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed). 2011;16:74–104. Review.
Morrison SF, Madden CJ. Central nervous system regulation of brown adipose tissue. Compr Physiol. 2014;4(4):1677–713. Review.
Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64(1):35–46.
Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91(2):389–411. Review.
Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, et al. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48(9):1787–93.
Enriori PJ, Sinnayah P, Simonds SE, Garcia RC, Cowley MA. J Neurosci. 2011;31:12189.
Pellegrino MJ, McCully BH, Habecker BA. Leptin stimulates sympathetic axon outgrowth. Neurosci Lett. 2014;566:1–5. Demonstrates leptin’s ability to stimulate axonal growth of sympathetic neurons in vitro.
Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163(1):84–94. Shows leptin-mediated direct synapsing of sympathetic neurons on white adipose tissue in vivo.
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–4.
Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495(7441):379–83.
Townsend KL, Suzuki R, Huang TL, Jing E, Schulz TJ, Lee K, et al. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 2012;26(5):2187–96.
Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149(4):871–85.
Lipton JM, Glyn JR. Central administration of peptides alters thermoregulation in the rabbit. Peptides. 1980;1(1):15–8.
Oldfield BJ, Allen AM, Davern P, Giles ME, Owens NC. Lateral hypothalamic ‘command neurons’ with axonal projections to regions involved in both feeding and thermogenesis. Eur J Neurosci. 2007;25(8):2404–12.
Kasahara Y, Sato K, Takayanagi Y, Mizukami H, Ozawa K, Hidema S, et al. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology. 2013;154(11):4305–15.
Kasahara Y, Takayanagi Y, Kawada T, Itoi K, Nishimori K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Biosci Biotechnol Biochem. 2007;71(12):3122–6.
Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20(4):670–7. Provide the mechanism by which pharmacologically delivered FGF21 acts centrally to increase energy expenditure.
Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28(12):2382–6.
Yang C, Jin C, Li X, Wang F, McKeehan WL, Luo Y. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One. 2012;7(3):e33870.
Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59(7):1817–24.
Tan BK, Hallschmid M, Adya R, Kern W, Lehnert H, Randeva HS. Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid: relationship with plasma FGF21 and body adiposity. Diabetes. 2011;60(11):2758–62.
Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19(9):1147–52.
Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat—functional implications. J Clin Invest. 2002;110(9):1243–50.
Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1243–55.
De Matteis R, Ricquier D, Cinti S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J Neurocytol. 1998;27(12):877–86.
Ruschke K, Ebelt H, Klöting N, Boettger T, Raum K, Blüher M, et al. Defective peripheral nerve development is linked to abnormal architecture and metabolic activity of adipose tissue in Nscl-2 mutant mice. PLoS One. 2009;4(5):e5516.
Wan Y, Xue R, Wang Y, Zhang Q, Huang S, Wu W, et al. The effect of neuropeptide Y on brown-like adipocyte’s differentiation and activation. Peptides. 2015;63:126–33.
Yang K, Guan H, Arany E, Hill DJ, Cao X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 2008;22(7):2452–64.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Magdalena Blaszkiewicz and Kristy L. Townsend declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Obesity Treatment
Rights and permissions
About this article
Cite this article
Blaszkiewicz, M., Townsend, K.L. Adipose Tissue and Energy Expenditure: Central and Peripheral Neural Activation Pathways. Curr Obes Rep 5, 241–250 (2016). https://doi.org/10.1007/s13679-016-0216-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-016-0216-9