Abstract
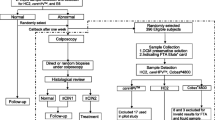
Cell profiles determined by the thin-layer advanced cytology assay system (TACAS™), a liquid-based cytology technique newly developed in Japan, were analyzed in this study. Hybrid capture 2 (HC-2) was also performed using the liquid-based samples prepared by TACAS to ascertain its ability to detect human papillomavirus (HPV). Cell collection samples from uterine cervix were obtained from 359 patients and examined cytologically. A HC-2 assay for HPV was carried out in the cell specimens. All specimens were found to show background factors such as leukocytes. After excluding the 5 unsatisfactory cases from the total 354 cases, 82 cases (23.2%) were positive and 272 cases (76.8%) were negative for HPV. Cell specimens from 30 HPV-positive cases and 166 HPV-negative cases were subjected to 4 weeks of preservation at room temperature. Then, when subsequently re-assayed, 28 cases (93.3%) in the former group were found to be HPV positive and 164 cases (98.8%) in the latter group were found to be HPV negative. These results supported the excellent reproducibility of TACAS for HPV testing. A reasonable inference from the foregoing analysis is that TACAS may be distinguished from other liquid-based cytological approaches, such as ThinPrep and SurePath, in that it can retain the cell backgrounds. Furthermore, this study raises the possibility that cell specimens prepared using TACAS could be preserved for at least 4 weeks prior to carrying out a HC-2 assay for HPV.

Similar content being viewed by others
References
http://apps.who.int/hpvcentre/statistics/dynamic/ico/SummaryReportsSelect.cfm. Accessed 2 Dec 2010.
Konno R, Dobbelaere KO, Godeaux OO, et al. Immunogenicity, reactogenicity, and safety of human papillomavirus 16/18 AS04-adjuvanted vaccine in Japanese women: interim analysis of a phase II, double-blind, randomized controlled trial at month 7. Int J Gynecol Cancer. 2009;19:905–11.
Doyle B, O’Farrell C, Mahoney E, et al. Liquid-based cytology improves productivity in cervical cytology screening. Cytopathology. 2006;17:60–4.
Kirschner B, Simonsen K, Junge J. Comparison of conventional Papanicolaou smear and SurePath liquid-based cytology in the Copenhagen population screening programme for cervical cancer. Cytopathology. 2006;17:187–94.
Williams AR. Liquid-based cytology and conventional smears compared over two 12-month periods. Cytopathology. 2006;17:82–5.
Ronco G, Cuzick J, Pierotti P, et al. Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening: randomised controlled trial. BMJ. 2007;335:28.
Strander B, Andersson-Ellstrom A, Milsom I, et al. Liquid-based cytology versus conventional Papanicolaou smear in an organized screening program: a prospective randomized study. Cancer. 2007;111:285–91.
Bergeron C, Bishop J, Lemarie A, et al. Accuracy of thin-layer cytology in patients undergoing cervical cone biopsy. Acta Cytol. 2001;45:519–24.
Hessling JJ, Raso DS, Schiffer B, et al. Effectiveness of thin-layer preparations vs. conventional Pap smears in a blinded, split-sample study. Extended cytologic evaluation. J Reprod Med. 2001;46:880–6.
Schledermann D, Ejersbo D, Hoelund B. Improvement of diagnostic accuracy and screening conditions with liquid-based cytology. Diagn Cytopathol. 2006;34:780–5.
Ferenczy A, Robitaille J, Franco E, et al. Conventional cervical cytologic smears vs. ThinPrep smears. A paired comparison study on cervical cytology. Acta Cytol. 1996;40:1136–42.
Sherman ME, Mendoza M, Lee KR, et al. Performance of liquid-based, thin-layer cervical cytology: correlation with reference diagnoses and human papillomavirus testing. Mod Pathol. 1998;11:837–43.
Arbyn M, Bergeron C, Klinkhamer P, et al. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111:167–77.
Davey E, Barratt A, Irwig L, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122–32.
Siebers AG, Klinkhamer PJ, Grefte JM, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: a randomized controlled trial. JAMA. 2009;302:1757–64.
Masumoto N, Fujii T, Ishikawa M, et al. Papanicolaou tests and molecular analyses using new fluid-based specimen collection technology in 3000 Japanese women. Br J Cancer. 2003;88:1883–8.
Taoka H, Yamamoto Y, Sakurai N, et al. Comparison of conventional and liquid-based cytology, and human papillomavirus testing using SurePath preparation in Japan. Hum Cell. 2010;23:126–33.
Jamison J, Wilson RT, Carson J. The evaluation of human papillomavirus genotyping in cervical liquid-based cytology specimens; using the Roche Linear Array HPV genotyping assay. Cytopathology. 2009;20:242–8.
Lee JK, Kim MK, Song SH, et al. Comparison of human papillomavirus detection and typing by hybrid capture 2, linear array, DNA chip, and cycle sequencing in cervical swab samples. Int J Gynecol Cancer. 2009;19:266–72.
Hardie A, Moore C, Patnick J, et al. High-risk HPV detection in specimens collected in SurePath preservative fluid: comparison of ambient and refrigerated storage. Cytopathology. 2009;20:235–41.
Obwegeser JH, Brack S. Does liquid-based technology really improve detection of cervical neoplasia? A prospective, randomized trial comparing the ThinPrep Pap Test with the conventional Pap Test, including follow-up of HSIL cases. Acta Cytol. 2001;45:709–14.
Taylor S, Kuhn L, Dupree W, et al. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2006;118:957–62.
Sykes PH, Harker DY, Miller A, et al. A randomised comparison of SurePath liquid-based cytology and conventional smear cytology in a colposcopy clinic setting. BJOG. 2008;115:1375–81.
Beerman H, van Dorst EB, Kuenen-Boumeester V, et al. Superior performance of liquid-based versus conventional cytology in a population-based cervical cancer screening program. Gynecol Oncol. 2009;112:572–6.
Davey E, d’Assuncao J, Irwig L, et al. Accuracy of reading liquid based cytology slides using the ThinPrep Imager compared with conventional cytology: prospective study. BMJ. 2007;335:31.
Sweeney BJ, Haq Z, Happel JF, Weinstein B, Schneider D. Comparison of the effectiveness of two liquid-based Papanicolaou systems in the handling of adverse limiting factors, such as excessive blood. Cancer. 2006;108:27–31.
Wright TC Jr. Cervical cancer screening in the 21st century: is it time to retire the PAP smear? Clin Obstet Gynecol. 2007;50:313–23.
Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630–6.
Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–9.
Castle PE, Rodríguez AC, Burk RD, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi:10.1136/bmj.b2569.
http://www.asccp.org/pdfs/consensus/hpv_genotyping_20090320.pdf. Accessed 5 Dec 2010.
Acknowledgments
We thank Dr Toshiaki Oharaseki for pathological discussion, and the cytotechnologists, Mr. Katsuji Taguchi, Mr. Masashi Fujita, Ms. Yoshie Muraishi, and Ms. Tomoko Kawabata for cytoscreening. We also thank Ms. Miyuki Saito for her helpful discussion during this study.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubushiro, K., Taoka, H., Sakurai, N. et al. Newly developed liquid-based cytology. TACAS™: cytological appearance and HPV testing using liquid-based sample. Human Cell 24, 115–120 (2011). https://doi.org/10.1007/s13577-011-0020-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-011-0020-5