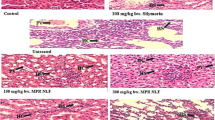
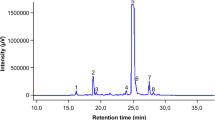
Abstract
Phyllanthus emblica (Euphorbiaceae) possesses a vast ethnomedical history and represents a phytochemical reservoir of heuristic medicinal value. Polyphenolic extract of P. emblica fruits, was investigated for antioxidant potential against lead acetate induced toxicity in female Wistar rats. The liver of female Wistar rats treated with lead acetate at different concentrations showed significant increase in lipid peroxidation and decrease in activities of superoxide dismutase and catalase. Furthermore, lead acetate also caused decrease in reduced glutathione content in rat liver. However, the co-incubation of extract and lead acetate showed significant protection in concentration-dependent manner. Furthermore, the polyphenolic extract of P. emblica fruits also found to have potent free radical scavenging activity as assessed by reducing power assay, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical (IC50 15.5 μg/mL), superoxide radical (IC50 26 μg/mL) and hydrogen peroxide scavenging assays (IC50 22 μg/mL) under in vitro conditions. Moreover the phytochemical characterization of the extract was also measured by determining total phenolic, flavonoids and tannin contents. This activity of the extract may be due to the total polyphenolic contents present in it. The present study concluded that a polyphenolic rich fraction of P. emblica fruits is a potential source of natural antioxidants and thus could prevent oxidative stress induced by heavy metal toxicants.
Similar content being viewed by others
References
Reglero, M. M., Taggart, M. A., Monsalve-González, L. & Mateo, R. Heavy metal exposure in large game from a lead mining area: effects on oxidative stress and fatty acid composition in liver. Environ. Pollut. 157, 1388–1395 (2009).
Ademuyiwa, O. et al. Erythrocyte acetylcholinesterase activity as a surrogate indicator of lead-induced neurotoxicity in occupational lead exposure in Abeokuta, Nigeria. Environ. Toxicol Pharmacol. 24, 183–188 (2007).
Park, S. K. et al. Low-level lead exposure, metabolic syndrome, and heart rate variability: the VA Normative Aging Study. Environ. Health Perspect. 114, 1718–1724 (2006).
Patrick, L. Lead toxicity part II: the role of free radical damage and the use of 432 antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev. 433, 114–127 (2006).
El-Nekeety, A. A., El-Kady, A. A., Soliman, M. S., Hassan, N. S. & Abdel-Wahhab, M. A. Protective effect of Aquilegia vulgaris (L.) against lead acetateinduced oxidative stress in rats. Food Chem. Toxicol. 47, 2209–2215 (2009).
Perianayagam, J., Sharma, S., Joseph, A. & Christina, A. Evaluation of anti-pyretic and analgesic activity of Emblica officinalis. J. Ethnopharmacol. 95, 83–85 (2004).
Jose, J. K. & Kuttan, R. Antioxidant activity of Emblica officinalis. J. Clinical Biochem. Nut. 19, 63–70 (1995).
Jose, J. K., Kuttan, G. & Kuttan, R. Antitumor activity of Emblica officinalis. J. Ethnopharmacol. 75, 65–69 (2001).
Singh, I., Soyal, D. & Goyal, P. K. Emblica officinalis (Linn.) fruit extract provides protection against radiation-induced hematological and biochemical alterations in mice. J. Env. Pathol. Toxicol. Oncol. 25, 643–654 (2006).
Sultana, S., Ahmed, S. & Jahangir, T. Emblica officinalis and hepatocarcinogenesis: a chemopreventive study in Wistar rats. J. Ethnopharmacol. 118, 1–6 (2008).
Khan, M. T. et al. Identification of pyrogallol as an antiproliferative compound present in extracts from the medicinal plant Emblica officinalis: effects on in vitro cell growth of human tumor cell lines. Int. J. Oncol. 21, 187–192 (2002).
Al-Rehaily, A. J., Al-Howiriny, T. A., Al-Sohaibani, M. O. & Rafatullah, S. Gastroprotective effects of ‘Amla’ Emblica officinalis on in vivo test models in rats. Phytomedicine 9, 515–522 (2002).
Juang, L. J., Sheu, S. J. & Lin, T. C. Determination of hydrolyzable tannins in the fruit of Terminalia chebula by high-performancc liquid chromatography and capillary electrophoresis. J. Seperation Sci. 27, 718–724 (2004).
Ghosh, A. & Sil, P. C. A protein from Cajanus indicus Spreng protects liver and liver against mercuric chloride-induced oxidative stress. Biol. Pharm. Bull. 31, 1651–1658 (2008).
Hamadouche, N. A., Slimani, M., Merad-Boudia, B. & Zaoui, C. Reproductive toxicity of lead acetate in adult male rats. Am. J. Sci. Res. 3, 38–50 (2009).
Jin, Y. et al. Health effects in children aged 3–6 years induced by environmental lead exposure. Ecotoxicol. Environ. Saf. 63, 313–317 (2006).
Hiraganahalli, B. D. et al. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn. Mag. 8, 116–123 (2012).
Newairy, A. A. & Abdou, H. M. Protective role of flax lignans against lead acetate- induced oxidative damage and hyperlipidemia in rats. Food Chem. Toxicol. 47, 813–818 (2009).
Ohata, H., Kitauchi, S., Yoshimura, N., Mugitani, K. & Iwane, M. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. J. Cancer 109, 138–143 (2004).
Balasubashini, M. S., Rukkumani, R., Viswanathan, P. & Menon, V. P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. 18, 310–314 (2004).
Anuradha, R. & Palaniyandi, K. Antioxidant activity of methanolic extract of Pongamia pinnata on lead acetate induced hepatic damage in rats. Af. J. Biochem. Res. 5, 348–351 (2011).
Waraporn, Y., Athikom, S. & Roongtawan, S. Suppression of human fibrosarcoma cell metastasis by Phyllanthus emblica extract in vitro. Asian Pac. J. Cancer Prev. 14, 6863–6867 (2013).
Wiegand, H., Wagner, A. E. & Boesch-Saadatmandi, C. Effect of dietary genistein on Phase II and antioxidant enzymes in rat liver. Cancer Genomics Proteomics 6, 85–92 (2009).
Moon, H. K., Yang, E. S. & Park, J. W. Protection of peroxynitrite-induced DNA damage by dietary antioxidants. Arch. Pharmacol. Res. 29, 213–217 (2006).
Sivaramakrishnan, V., Narasimha, P., Shilpa, M., Kumar, V. R. & Devaraj, S. N. Attenuation of N-nitrosodie-thylamine-induced hepatocellular carcinogenesis by a novel flavonol Morin. Chem. Biol. Interac. 171, 79–88 (2008).
Dinkova-Kostova, A. T. Protection against cancer by plant phenyl propenoids: induction of mammalian anticarcinogenic enzymes. Mini Rev. Med. Chem. 2, 595–610 (2002).
Mylroie, A. A, Umbles, C. & Kyle, J. Effects of dietary copper supplementation on erythrocyte superoxide dismutase activity, ceruloplasmin and related parameters in rats ingesting lead acetate. Trace Subst. Environ. Health 18, 497–504 (1984).
Sharma S. K., Arogya, S. M., Bhaskarmurthy, D. H., Agarwal, A. & Velusami, C. C. Hepatoprotective activity of the Phyllanthus species on tert-butyl hydroperoxide (t-BH)-induced cytotoxicity in HepG2 cells. Pharmacogn. Mag. 7, 229–233 (2011).
Jindal, A., Soyal, D., Sharma, A. & Goyal, P. K. Protective effect of an extract of Emblica officinalis against radiation-induced damage in mice. Integr. Cancer Ther. 8, 98–105 (2009).
Rehman, H. et al. Studies on the chemical constituents of Phyllanthus emblica. Nat. Prod. Res. 21, 775–781 (2007).
Ross, W. D. Relationship of body mass index with skinfolds, girths, and bone breadths in Canadian men and women aged 20–70 year. Am. J. Phys. Anthrop. 77, 169–173 (1988).
Koriem, K. M. M. Lead toxicity and the protective role of Cupressus sempervirens. Rev. Latinoamer. Quím. 37, 230–242 (2009).
Sultana, S., Ahmad, S., Khan, N. & Jahangir, T. Effect of Emblica officinalis (Gaertn) on CCl4 induced hepatic toxicity and DNA synthesis in Wistar rats. Ind. J. Exp. Biol. 43, 430–436 (2005).
Ghosal, S., Tripathi, V. K. & Chauhan, S. Active constituent of Emblica officinalis: part 1st the chemistry and antioxidant effects of two new hydrolysable tannins, emblicanin A and B. Ind. J. Chem. 35, 941–948 (1996).
Bhattacharya, A., Chatterjee, A., Ghosal, S. & Bhattacharya, S. K. Antioxidant activity of active tannoid principles of Emblica officinalis (amla). Ind. J. Exp. Biol. 37, 676–680 (1999).
Alasalvar, C., Karamac, M., Amarowicz, R. & Shahidi, F. Antioxidant and antiradical activities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnut green leafy cover. J. Agri. Food Chem. 54, 4826–4832 (2006).
Li, H., Hao, Z., Wang, X., Huang, L. & Li, J. Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance. Biores. Tech. 100, 970–974 (2009).
Liu, X., Zhao, M., Wang, J., Yang, B. & Jiang, Y. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J. Food Comp. Anal. 21, 219–228 (2008).
Kiselova, Y. et al. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother. Res. 20, 961–965 (2006).
Meyer, A. S. & Isaksen, A. Application of enzymes as food antioxidants. Trends Food Sci. Technol. 6, 300–304 (1995).
Kumar, S., Suresh, P. K., Vijayababu, M. R., Arunkumar, A. & Arunakaran, J. Anticancer effects of ethanolic neem leaf extract on prostate cancer cell line (PC-3). J. Ethnopharmacol. 105, 246–250 (2006).
Shahidi, F., Alasalvar, C. & Liyana-Pathirana, C. M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproduct. J. Agri. Food Chem. 55, 1212–1220 (2007).
Kumarappan, C. T., Thilagam, E. & Mandal, S. C. Antioxidant activity of polyphenolic extracts of Ichnocarpus frutescens. Saudi J. Biol. Sci. 19, 349 (2012).
Sofowara, A. in Medicinal Plants and Traditional Medicine in Africa (Spectrum Books Ltd., Nigeria, 2009).
Singleton, V. L., Orthofer, R. & Lamuela-Raventos, R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Cioalteau reagent. Methods Enzymol. 299, 152–178 (1999).
Meda, A., Lamien, C. E., Romito, M., Millogo, J. & Nacoulma, O. G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 91, 571–577 (2005).
Hagerman, A. E. & Butler, L. G. Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 26, 809–812 (1978).
Ohkawa, H., Ohishi, N. & Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358 (1979).
Kakkar, P., Das, B. & Viswanathan, P. N. A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys. 21, 130–132 (1984).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with folin-phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Sinha, A. K. Calorimetric assay of catalase. Anal. Biochem. 47, 389–394 (1972).
Moron, M. S., Depierre, J. W. & Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem. Biophys. Acta. 582, 67–78 (1979).
Jayaprakash, G. K., Singh, R. P. & Sakariah, K. K. Antioxidant activity of grape seed extracts on peroxidation models in vitro. J. Agric. Food Chem. 55, 1018 (2001).
Shimada, K., Fujikawa, K., Yahara, K. & Nakamura, T. Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 40, 945–948 (1992).
Liu, F., Ooi, V. E. & Chang, S. T. Free radical scavenging activity of mushroom polysaccharide extracts. Life Sci. 60, 763–771 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saha, S., Verma, R.J. Antioxidant activity of polyphenolic extract of Phyllanthus emblica against lead acetate induced oxidative stress. Toxicol. Environ. Health Sci. 7, 82–90 (2015). https://doi.org/10.1007/s13530-015-0224-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-015-0224-2