Abstract
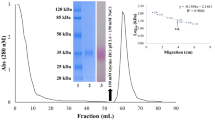
The green rice leafhopper, Nephotettix cincticeps (Uhler), is an insect pest of rice and discharges β-glucosidase (EC 3.2.1.21) from its salivary glands during feeding. To investigate the biological function of this enzyme, we purified it from the heads of 18,000 adult females by acetone precipitation and a series of chromatography steps: gel filtration, cation-exchange chromatography, metal-affinity chromatography and hydrophobic interaction chromatography. During cation-exchange chromatography, β-glucosidases were eluted in three peaks (isozymes). These β-glucosidases were monomeric proteins of 58 kDa as estimated by SDS-PAGE and 62 kDa based on gel filtration. All of the purified β-glucosidase isozymes exhibited maximum activity for p-nitrophenyl β-glucoside (NPGlc) and p-nitrophenyl β-galactopyranoside (NPGal) at pH 5.5 and 5.0, respectively. There was no significant difference in substrate specificity among the three isozymes. The Km values were estimated to be 0.13 μM for NPGlc and 0.9 μM for NPGal. Among the oligosaccharide substrates examined, laminaribiose (Glc β1-3 Glc) was the most extensively hydrolyzed, sophorose (Glc β1-2 Glc) and cellobiose (Glc β1-4 Glc) were comparatively well hydrolyzed, and gentiobiose (Glc β1-6 Glc), lactose (Gal β1-4 Glc), laminaritriose, cellotriose and cellotetraose were poorly hydrolyzed. Among the glycoside substrates examined, salicin was considerably well hydrolyzed. β-Glucosidase was detected in the salivary sheaths by activity staining with a fluorescent substrate. The salivary β-glucosidase of N. cincticeps may be involved in the hydrolysis of a phenol glucoside present in the saliva, which is a step in the solidification of gelling saliva to form salivary sheaths.







Similar content being viewed by others
References
Baker JE, Woo SM (1992) β-Glucosidases in the rice weevil, Sitophilus oryzae: purification, properties, and activity levels in wheat- and legume-feeding strains. Insect Biochem Mol Biol 22:495–504
Britton HTS, Robinson RA (1931) Universal buffer solutions and the dissociation constant of veronal. J Chem Soc 1456–1462
Chararas C, Chipoulet JM (1982) Purification by chromatography and properties of a β-glucosidase from the larvae of Phoracantha semipunctata. Comp Biochem Phys B 72:559–564
Ferreira C, Terra WR (1983) Physical and kinetic properties of a plasmamembrane-bound, β-D-glucosidase (cellobiase) from midgut cells of an insect (Rhynchosciara americana larva). Biochem J 213:43–51
Ferreira C, Parra JRP, Terra WR (1997) The effect of dietary plant glycosides on larval midgut β-glucosidases from Spodoptera frugiperda and Diatraea saccharalis. Insect Biochem Mol Biol 27:55–59
Ferreira C, Torres BB, Terra WR (1998) Substrate specificities of midgut β-glucosidases from insects of different orders. Comp Biochem Phys B 119:219–225
Ferreira AHP, Marana SR, Terra WR, Ferreira C (2001) Purification, molecular cloning, and properties of a β-glucosidase isolated from midgut lumen of Tenebrio molitor (Coleoptera) larvae. Insect Biochem Mol Biol 31:1065–1076
Hattori M, Konishi H, Tamura Y, Konno K, Sogawa K (2005) Laccase-type phenoloxidase in salivary glands and watery saliva of the green rice leafhopper, Nephotettix cincticeps. J Insect Physiol 51:1359–1365
Hattori M, Tsuchihara K, Konishi H, Tamura Y, Noda H, Shinoda T, Sogawa K (2010) Molecular characterization and expression of laccase genes in the salivary glands of the green rice leafhopper, Nephotettix cincticeps (Hemiptera: Cicadellidae). Insect Biochem Mol Biol 40:331–338
Hibino H (1996) Biology and epidemiology of rice viruses. Annu Rev Phytopathol 34:249–274
Kawabe S (1985) Mechanism of varietal resistance to the rice green leafhopper (Nephotettix cincticeps Uhler). JARQ 19:115–124
Kawabe S (1994) Development of a method for analysis of feeding and transmitting behavior in Homopteran insects. Kenkyu Seika 295:52–63 (in Japanese)
Marana SR, Terra WR, Ferreira C (1995) Midgut β-D-glucosidases from Abracris flavolineata (Orthoptera: Acrididae). Physical properties, substrate specificities and function. Insect Biochem Mol Biol 25:835–843
Marana SR, Terra WR, Ferreira C (2000) Purification and properties of a β-glucosidase purified from midgut cells of Spodoptera frugiperda (Lepidoptera) larvae. Insect Biochem Mol Biol 30:1139–1146
McIlvaine TC (1921) A buffer solution for colorimetric comparison. J Biol Chem 49:183–186
Miles PW (1999) Aphid saliva. Biol Rev Camb Philos Soc 74:41–85
Morgan MRJ (1975) Relationship between gut cellobiase, lactase, aryl β-glucosidase, and aryl β-galactosidase activities of Locusta migratoria. Insect Biochem 5:609–611
Nakashima K, Kato S, Iwanami S, Murata N (1991) Cloning and detection of chromosomal and extrachromosomal DNA from mycoplasmalike organisms that cause yellow dwarf disease of rice. Appl Environ Microbiol 57:3570–3575
Pontoh J, Low NH (2002) Purification and characterization of β-glucosidase from honey bees (Apis mellifera). Insect Biochem Mol Biol 32:679–690
Pratviel F, Clermont S, Percheron F, Chararas C (1987) Studies on glycosidases and glucanases in Thaumetopoea pityocampa larvae. II. Purification and some properties of a broad specificity β-D-glucosidase. Comp Biochem Phys B 86:173–178
Sakoda M, Hiromi K (1976) Determination of the best-fit values of kinetic parameters of the Michaelis-Menten equation by the method of least squares with the Taylor expansion. J Biochem 80:547–555
Santos CD, Terra WR (1985) Physical properties, substrate specificities and a probable mechanism for a β-D-glucosidase (cellobiase) from midgut cells of the cassava hornworm (Erinnyis ello). Biochem Biophys Acta 831:179–185
Sogawa K (1968a) Studies on the salivary glands of rice plant leafhoppers. III. Salivary phenolase. Appl Entomol Zool 3:13–25
Sogawa K (1968b) Studies on the salivary glands of rice plant leafhoppers. IV. Carbohydrate activities. Appl Entomol Zool 3:67–73
Sogawa K (1971) Studies on the salivary glands of rice plant leafhoppers. V. Formation of the stylet sheath. Jpn J Appl Entomol Zool 15:132–138 (in Japanese with English summary)
Sogawa K (1973) Feeding of the rice plant-and leafhoppers. Rev Plant Prot Res 6:31–43
Terra WR, Ferreira C (1994) Insect digestive enzymes: properties, compartmentalisation and function. Comp Biochem Physiol 109B:1–62
Terra WR, Ferreira C (2005) Biochemistry of digestion. In: Gilbert LI, Iatrou K, Gill SS (eds) Comprehensive Molecular Insect Science, vol 4. Elsevier, Oxford, pp 171–224
Terra WR, Ferreira C, Jordao BP, Dillon RJ (1996) Digestive enzymes. In: Lehane MJ, Billingsley PF (eds) Biology of the Insect Midgut. Chapman and Hall, London, pp 153–194
Tokuda G, Watanabe H, Matsumoto T, Noda H (1997) Cellulose digestion in the wood-eating higher termite, Nasutitermes takasagoensis (Shiraki): distribution of cellulases and properties of endo-h-1,4- glucanase. Zool Sci 14:83–93
Tokuda G, Saito H, Watanabe H (2002) A digestive β-glucosidase from the salivary glands of the termite, Neotermes koshunensis (Shiraki): distribution, characterization and isolation of its precursor cDNA by 5′- and 3′-RACE amplifications with degenerate primers. Insect Biochem Mol Biol 32:1681–1689
Tokuda G, Miyagi M, Makiya H, Watanabe H, Arakawa G (2009) Digestive β-glucosidases from the wood-feeding higher termite, Nasutitermes takasagoensis: intestinal distribution, molecular characterization, and alteration in sites of expression. Insect Biochem Mol Biol 39:931–937
Urano Y, Kamiya M, Kanda K, Ueno T, Hirose K, Nagano T (2005) Evolution of fluorescein as a platform for finely tunable fluorescence probes. J Am Chem Soc 127:4888–4894
Whetten R, Sederoff R (1995) Lignin biosynthesis. Plant Cell 7:1001–1013
Yapi DYA, Gnakri D, Niamke SL, Kouame LP (2009) Purification and biochemical characterization of a specific β-glucosidase from the digestive fluid of larvae of the palm weevil, Rhynchophorus palmarum. J Insect Sci 9:1–13
Yu SJ (1989) β-Glucosidase in four phytophagous Lepidoptera. Insect Biochem 19:103–108
Acknowledgments
We thank M. Watanabe for technical assistance, and K. Hashino and H. Niki for assistance with rearing and dissecting insects. We are grateful to Y. Tamura and T. Hasegawa for their valuable comments on the study. This work was partly supported by JSPS KAKENHI grant no. 22580063 and 25450075 to M.H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, M., Hattori, M. Purification of β-glucosidase from the salivary glands of the green rice leafhopper, Nephotettix cincticeps (Uhler) (Hemiptera: Cicadellidae), and its detection in the salivary sheath. Appl Entomol Zool 48, 489–497 (2013). https://doi.org/10.1007/s13355-013-0210-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-013-0210-6