Abstract
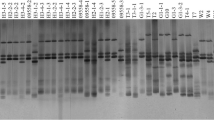
Spot blotch, caused by Bipolaris sorokiniana, is a prevalent disease of barley in Canada and elsewhere in the world. To evaluate the genetic variation among pathogen isolates, 93 isolates of B. sorokiniana representative of eight different virulence groups (VIGs) identified in a previous study, were subjected to an AFLP assay. AFLP analysis indicated that pathogen isolates collected from different regions of Canada and from other countries had a high level of genetic variability. Isolates possessing low virulence (VIG 0.0.0.0) and differential virulence (VIG 6.0.0.0) on barley genotypes were clearly discernible from other pathogenic isolates. However, molecular analysis did not provide a robust differentiation among the other six VIGs identified by the classical method of pathotype designation. There was a closer correlation between the AFLP patterns and virulence than between AFLP pattern and geographic origin of isolates. To evaluate causes of genetic variation among isolates, the influence of mutation, migration and gene flow, and recombination on the B. sorokiniana population were discussed. The genetic profiles of the three isolates of VIG 6.0.0.0, which were located between low virulence and high virulence isolates, invited speculation that such a virulence group may be the result of genetic recombination between isolates possessing extreme virulences in the population of the pathogen.


Similar content being viewed by others
References
Asigbetse KB, Fernandez D, Dubois MP, Geiger JP (1994) Differentiation of Fusarium oxysporum f. sp. vasinfectum races on cotton by random amplified polymorphic DNA analysis. Phytopathology 84:622–626
Bulat SA, Mironenko NV (1993) Genetic differentiation of the phytopathogenic fungus Cochliobolus sativus (Ito and Kurib.) Drechsl. ex Dastur (Bipolaris sorokiniana (Sacc.: Sorok.) Shoem.) revealed by a universally primed polymerase chain reaction UP-PCR technique: Correlation with host-specificity. (In Russian). Genetika 29:1295–1301
Burdon JJ, Silk J (1997) Sources and patterns of diversity in plant pathogenic fungi. Phytopathology 87:664–669
Burdon JJ, Roelfs AP (1985a) Isozyme and virulence variation in asexually reproducing populations of Puccinia graminis and P. recondita on wheat. Phytopathology 75:907–913
Burdon JJ, Roelfs AP (1985b) The effect of sexual and asexual reproduction on the isozyme structure of populations of Puccinia graminis. Phytopathology 75:1068–1073
Chand R, Pandey SP, Singh HV, Kumar S, Joshi AK (2003) Variability and its probable causes in natural populations of spot blotch pathogen Bipolaris sorokiniana of wheat (T. aestivum L.) in India. Z Pflanzenkr Pflanzenschutz 110:27–35
Christensen JJ, Davies FR (1937) Nature of variation in Helminthosporium sativum. Mycologia 29:85–99
Clark RV (1979) Yield losses in barley cultivars caused by spot blotch. Can J Plant Pathol 2:113–117
de Moura Nascimento EJ, Van Der Sand ST (2008) Restriction analysis of the amplified ribosomal DNA spacers ITS1 and ITS2 of Bipolaris sorokiniana isolates. World J Microbiol Biotechnol 24:647–652
de Oliveira AM, Matsumura AT, Prestes AM, Van Der Sand ST (2002) Intraspecific variability of Bipolaris sorokiniana isolates determined by random-amplified polymorphic DNA (RAPD). Genet Mol Res 1:350–358
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26:297–302
Dostaler D, Couture L, Pelletier GJ (1987) Étude de la tolérance de cultivars d’ orge à la tache helminthosporienne. Can J Plant Sci 67:153–157
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fetch TG, Steffenson BJ (1994) Identification of Cochliobolus sativus isolates expressing differential virulence on two-row barley genotypes from North Dakota. Can J Plant Pathol 16:202–206
Fordyce M, Meldrum S (2001) Verification of conventional pathotype screening for Australian isolates of Cochliobolus sativus using molecular fingerprinting. In: Proc of the 10th Australian Barley Technical Symposium, 16–20 September 2001. Canberra, Australia. [Online article].
Ghazvini H, Tekauz A (2004) Yield loss in barley inoculated with high and low virulence isolates of Bipolaris sorokiniana. In: Proc of the 9th International Barley Genetic Symposium, June 20–26, 2004, Brno, Czech Republic, pp. 774–780
Ghazvini H, Tekauz A (2007) Virulence diversity in the population of Bipolaris sorokiniana. Plant Dis 91:814–821
Ghazvini H, Tekauz A (2008) Host–pathogen interactions among barley genotypes and Bipolaris sorokiniana isolates. Plant Dis 92:225–233
Harding H, Tinline RD (1983) The existence of differentially fertile strains in two populations of Cochliobolus sativus. Can J Plant Pathol 5:17–20
Hosford RM Jr, Solangi GRM, Kiesling RL (1975) Inheritance in Cochliobolus sativus. Phytopathology 65:699–703
Knight NL, Platz GJ, Lehmensiek A, Sutherland MW (2010) An investigation of genetic variation among Australian isolates of Bipolaris sorokiniana from different cereal tissues and comparison of their abilities to cause spot blotch on barley. Australas Plant Pathol 39:207–216
Kolmer JA (2001) Molecular polymorphism and virulence phenotypes of the wheat leaf rust fungus Puccinia triticina in Canada. Can J Bot 79:917–926
Kolmer JA, Liu JQ, Sies M (1995) Virulence and molecular polymorphism in Puccinia recondita f. sp. tritici in Canada. Phytopathology 85:276–285
Leisova-Svobodova L, Minarikova V, Kucera L, Pereyra SA (2011) Structure of the Cochliobolus sativus population variability. Plant Pathol. doi:10.1111/j.1365-3059.2011.02547.x
Levy M, Romao J, Marchetti MA, Hamer JE (1991) DNA fingerprinting with a dispersed repeated sequence resolves pathotype diversity in the rice blast fungus. Plant Cell 3:95–102
Levy M, Correa-Victoria FJ, Zeigler RS, Xu S, Hamer JE (1993) Genetic diversity of the rice blast fungus in a disease nursery in Columbia. Phytopathology 83:1427–1433
Limpert E, Muller K (1994) Designation of pathotypes of plant pathogens. J Phytopathol 140:346–358
Liu JQ, Kolmer JA (1998) Molecular and virulence diversity and linkage disequilibria in asexual and sexual populations of the wheat leaf rust fungus, Puccinia recondita. Genome 41:832–840
Majer D, Mithen R, Lewis BG, Vos P, Oliver RP (1996) The use of AFLP fingerprinting for the detection of variation in fungi. Mycol Res 100:1107–1111
McDonald BA, McDermott JM, Goodwin SB, Allard RW (1989) The population biology of host-pathogen interactions. Annu Rev Phytopathol 27:77–94
Meldrum SI, Ogle HJ, Platz GJ (2004) Pathotypes of Cochliobolus sativus on barley in Australia. Australas Plant Pathol 33:109–114
Michelmore RW, Hulbert SH (1987) Molecular markers for genetic analyses of phytopathogenic fungi. Annu Rev Phytopathol 25:383–404
Mironenko NV, Bulat SA (2001) Genetic structure of Cochliobolus sativus (Bipolaris sorokiniana) populations isolated from different hosts as revealed by UP-PCR (RAPD-like) technique. J Russ Phytopathol Soc 2:25–30
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Pongam P, Osborn CT, Williams PH (1999) Assessment of genetic variation among Leptosphaeria maculans isolates using pathogenicity data and AFLP analysis. Plant Dis 83:149–154
Posada D, Crandall KA, Holmes EC (2002) Recombination in evolutionary genomics. Annu Rev Genet 36:75–97
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238
Raemaekers RH (1988) Helminthosporium sativum: disease complex on wheat and sources of resistance in Zambia. In: Klatt AR (ed) Wheat production constraints in tropical environments. CIMMYT, Mexico, DF, Mexico, pp 175–185
Rau D, Brown AHD, Brubaker CL, Attene G, Balmas V, Saba E, Papa R (2003) Population genetic structure of Pyrenophora teres Drechs. the causal agent of net blotch in Sardinian landraces of barley (Hordeum vulgare L.). Theor Appl Genet 106:947–959
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Shoemaker RA (1955) Biology, cytology and taxonomy of Cochliobolus sativus. Can J Bot 33:562–576
Simcox KD, Nickrent D, Pedersen WL (1992) Comparison of isozyme polymorphism in races of Cochliobolus carbonum. Phytopathology 82:621–624
Sneath PHA, Sokal RR (1973) Numerical taxonomy. Freeman, San Francisco, p 573
Swofford DL (2002) PAUP* phylogenetic analysis using parsimony and other programs, version 4.0b10. Sinauer Associates, Sunderland
Swofford DL, Olsen GJ, Waddell PJ, Hillis DM (1996) Phylogenetic inference. In: Hillis DM, Moritz C, Mable BK (eds) Molecular systematics, 2nd edn. Sinauer Associates, Sunderland, pp 407–514
Tekauz A. 2002. Spot blotch (Cochliobolus sativus) infection responses in selected North American barley cultivars at the seedling and adult-plant stages. In: Proc. 2nd Int. Workshop Barley Leaf Blights. ICARDA, Aleppo, Syria, pp. 428–435
Terekhova VA, Rochev MV (1989) Comparative characterization of mycelial isozymes of Bipolaris sorokiniana Shoem. isolates of different origin. Moscow Univ Biol Sci Bull 44:77–81
Tinline RD (1951) Studies of the perfect stage of Helminthosporium sativum. Can J Bot 29:467–478
Tinline RD (1961) Cochliobolus sativus. IV. Drug-resistant, color, and nutritionally exacting mutants. Can J Bot 39:1695–1704
Tinline RD (1962) Cochliobolus sativus. V. Heterokariosis and parasexuality. Can J Bot 40:425–437
Tinline RD (1988) Cochliobolus sativus, a pathogen of wide host range. In: Ingram DS, Williams PH (eds) Advances in plant pathology, vol 6. Academic, London, pp 113–122
Tinline RD, Dickson JG (1958) Cochliobolus sativus. I. Perithecial development and inheritance of spore color and mating type. Mycologia 50:697–706
Valim-Labres ME, Porto MD, Matsumura ATS (1997) Effects of host resistance on the isozymatic patterns of Bipolaris sorokiniana (Dematiaceae, Moniliales). Braz J Genet 20:541–545
Valjavec-Gratian M, Steffenson BJ (1997) Pathotypes of Cochliobolus sativus on barley. Plant Dis 81:1275–1278
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucl Acid Res 23:4407–4414
Welz HG, Kohler W, Leonard KJ (1994) Isozyme variation within and among pathogenic races of Cochliobolus carbonum on corn in North Carolina. Phytopathology 84:31–38
Yoder OC (1988) Cochliobolus heterostrophus, cause of southern corn leaf blight. In: Ingram DS, Williams PH (eds) Advances in plant pathology, vol 6. Academic, London, pp 93–112
Zeigler RS, Cuoc LX, Scott RP, Bernardo MA, Chen DH, Valent B, Nelson RJ (1995) The relationship between lineage and virulence in Pyricularia grisea in the Philippines. Phytopathology 85:443–451
Zhong S, Steffenson BJ (2001) Virulence and molecular diversity in Cochliobolus sativus. Phytopathology 91:469–476
Zhong S, Steffenson BJ (2002) Identification and characterization of DNA markers associated with a locus conferring virulence on barley in the plant pathogenic fungus Cochliobolus sativus. Theor Appl Genet 104:1049–1054
Zhong S, Steffenson BJ (2007) Molecular karyotyping and chromosome length polymorphism in Cochliobolus sativus. Mycol Res 111:78–86
Acknowledgments
We wish to thank Dr. Bruce Ford, Dr. Michele Piercey-Normore and Dr. Anne Worley, Dept of Botany, University of Manitoba, for their valuable comments and suggestions and also the help they provided with data analysis of this study. We also would like to thank Leslie Bezte for her valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 179 kb)
Rights and permissions
About this article
Cite this article
Ghazvini, H., Tekauz, A. Molecular diversity in the barley pathogen Bipolaris sorokiniana (Cochliobolus sativus). Australasian Plant Pathol. 41, 283–293 (2012). https://doi.org/10.1007/s13313-012-0131-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-012-0131-9