Abstract
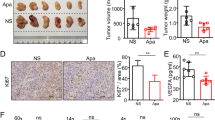
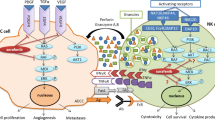
Sorafenib, a multi-tyrosine kinase inhibitor, is a standard treatment for advanced hepatocellular carcinoma (HCC). Herein, we report that the combinatorial therapy of sorafenib and anti-programmed death-ligand 1 (PD-L1) monoclonal antibody (mAb) can be implemented with good results for HCC. Cancer mouse models were used to evaluate therapeutic efficacy and examine the immunologic mechanisms of the sorafenib/anti-PD-L1 mAb therapy. The combined administration of sorafenib and anti-PD-L1 mAb into tumor-bearing mice generated potent immune responses resulting in the complete eradication or remarkable reduction of tumor growth. In some instances, the sorafenib/anti-PD-L1 mAb therapy induced long-lasting protection against tumor rechallenges. The results indicate that NK cells but not CD4T cells or CD8 cells mediated the therapeutic efficacy of this combinatorial therapy. The overall results suggest that immunotherapy consisting of the combination of sorafenib/anti-PD-L1 mAb could be a promising new approach for treating patients with HCC.

Similar content being viewed by others
References
Tanaka S, Arii S. Molecular targeted therapies in hepatocellular carcinoma. Semin Oncol. 2012;39(4):486–92.
Vinas A et al. Mapping of DNA sex-specific markers and genes related to sex differentiation in turbot (Scophthalmus maximus). Mar Biotechnol (NY). 2012;14(5):655–63.
Ng CK et al. Deciphering the Sox-Oct partner code by quantitative cooperativity measurements. Nucleic Acids Res. 2012;40(11):4933–41.
Coco C et al. Increased expression of CD133 and reduced dystroglycan expression are strong predictors of poor outcome in colon cancer patients. J Exp Clin Cancer Res. 2012;31:71.
Zhang W et al. Epigenetic inactivation of the canonical Wnt antagonist SRY-box containing gene 17 in colorectal cancer. Cancer Res. 2008;68(8):2764–72.
Scott EL, Brann DW. Estrogen regulation of Dkk1 and Wnt/beta-catenin signaling in neurodegenerative disease. Brain Res. 2013;1514:63–74.
Mok TS et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57.
Mitsudomi T et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8.
Zhou C et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced <i>PD-L1</i> mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42.
Failli V, Bachy I, Rétaux S. Expression of the LIM-homeodomain gene <i>Lmx1a</i> (<i>dreher</i>) during development of the mouse nervous system. Mech Dev. 2002;118(1):225–8.
Murray KD, Choudary PV, Jones EG. Nucleus- and cell-specific gene expression in monkey thalamus. Proc Natl Acad Sci. 2007;104(6):1989–94.
Fu W et al. Insights into HER2 signaling from step-by-step optimization of anti-HER2 antibodies. MAbs. 2014;6(4):978–90.
Hu S et al. Comparison of the inhibition mechanisms of adalimumab and infliximab in treating tumor necrosis factor α-associated diseases from a molecular view. J Biol Chem. 2013;288(38):27059–67.
Ferlay J et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
Fujimoto-Ouchi K et al. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol. 2007;59(6):795–805.
Bang Y-J et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Kute T et al. Development of Herceptin resistance in breast cancer cells. Cytometry A. 2004;57(2):86–93.
Lu Y et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst. 2001;93(24):1852–7.
Nagy P et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a Herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65(2):473–82.
Price‐Schiavi SA et al. Rat Muc4 (sialomucin complex) reduces binding of anti‐ErbB2 antibodies to tumor cell surfaces, a potential mechanism for Herceptin resistance. Int J Cancer. 2002;99(6):783–91.
Scaltriti M et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–38.
Park J-G et al. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 1990;50(9):2773–80.
Kim SY et al. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int J Oncol. 2008;32(1):89–95.
Cho H-S et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421(6924):756–60.
Knuefermann C et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22(21):3205–12.
Scagliotti GV et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51.
Li YM et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6(5):459–69.
Geyer CE et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43.
Konecny GE et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630–9.
Schnitt SJ. Breast cancer in the 21st century: neu opportunities and neu challenges. Mod Pathol. 2001;14(3):213–8.
Bass AJ et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41(11):1238–42.
Hussenet T et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One. 2010;5(1):e8960.
Yuan P et al. Sex determining region Y-Box 2 (SOX2) is a potential cell-lineage gene highly expressed in the pathogenesis of squamous cell carcinomas of the lung. PLoS One. 2010;5(2):e9112.
Tompkins DH et al. Sox2 activates cell proliferation and differentiation in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45(1):101–10.
Tompkins DH et al. Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS One. 2009;4(12):e8248.
Aksoy I et al. Sox transcription factors require selective interactions with Oct4 and specific transactivation functions to mediate reprogramming. Stem Cells. 2013;31(12):2632–46.
Therasse P et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–16.
Franklin MC et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5(4):317–28.
Agus DB et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–37.
Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol Cell Biol. 1999;19(10):6845–57.
Conflicts of interest
None
Funding
Heilongjiang Province Natural Science Fund Project (D207016) Fund project of Heilongjiang Province Education Office (11501250).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yun Wang, Hongxia Li and Qi Liang are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Y., Li, H., Liang, Q. et al. Combinatorial immunotherapy of sorafenib and blockade of programmed death-ligand 1 induces effective natural killer cell responses against hepatocellular carcinoma. Tumor Biol. 36, 1561–1566 (2015). https://doi.org/10.1007/s13277-014-2722-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2722-2