Abstract
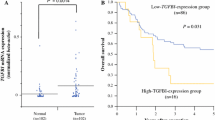
There is cumulative evidence that stromal reaction in cancer has an important diagnostic and prognostic significance. The aims of this study were to analyze the expression of basic fibroblast growth factor (FGF-2), CD31, and α-smooth muscle actin (SMA) in esophageal cancer patients and to establish their significance as indicators of disease recurrence after definitive chemoradiation (CRT). Protein expressions of FGF-2, CD31, and SMA were evaluated by immunohistochemistry and Western blot analysis in 70 patients, 20 with esophageal squamous cell carcinoma (ESCC) and 50 with locally recurrent ESCC after definitive CRT. Twenty matched normal esophageal squamous epithelium were also studied as controls. Esophageal cancer tissues showed positive expression of FGF-2, CD31, and SMA; in contrast, FGF-2 expression was not detected and only little staining for CD31 and SMA was noted in normal epithelium. Protein levels of FGF-2, CD31, and SMA were significantly elevated in recurrent ESCC. Among the patients with locally recurrent disease, expression of FGF-2 and SMA was notably high in whom the tumor recurred locally within 24 months after definitive CRT. The 2- and 5-year local recurrence-free survival rate was 15.4 % and 0 in patients with high FGF-2 expression, compared with 45.8 and 33.3 % in those who expressed low FGF-2, respectively (P = 0.005). Of patients who expressed high SMA, the 2- and 5-year local recurrence-free survival rate was 21.7 and 8.7 %, respectively, compared to those with low SMA expression which was 37.0 and 22.2 %, respectively (P = 0.016). Overexpression of FGF-2 and SMA is associated with local recurrence and reduced recurrence-free survival after definitive CRT for ESCC. The data also suggest that targeting stromal cells may be an attractive approach for esophageal cancer therapy strategies.




Similar content being viewed by others
References
Bremnes RM, Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, et al. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011;6:209–17.
Noma K, Smalley KS, Lioni M, Naomoto Y, Tanaka N, El-Deiry W, et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–93.
Xu Z, Wang S, Wu M, Zeng W, Wang X, Dong Z. TGFβ1 and HGF protein secretion by esophageal squamous epithelial cells and stromal fibroblasts in oesophageal carcinogenesis. Oncol Lett. 2013;6:401–6.
Barclay C, Li AW, Geldenhuys L, Baguma-Nibasheka M, Porter GA, Veugelers PJ, et al. Basic fibroblast growth factor (FGF-2) overexpression is a risk factor for esophageal cancer recurrence and reduced survival, which is ameliorated by coexpression of the FGF-2 antisense gene. Clin Cancer Res. 2005;11:7683–91.
Erez N, Truitt M, Olson P, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappa B-dependent manner. Cancer Cell. 2010;17:135–7.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401.
Hoff PM, Machado KK. Role of angiogenesis in the pathogenesis of cancer. Cancer Treat Rev. 2012;38:825–33.
GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. GLOBOCAN. IARC. 2013.
Chen W, He Y, Zheng R, Zhang S, Zeng H, Zou X, et al. Esophageal cancer incidence and mortality in China, 2009. J Thorac Dis. 2013;5:19–26.
Shridhar R, Almhanna K, Meredith KL, Biagioli MC, Chuong MD, Cruz A, et al. Radiation therapy and esophageal cancer. Cancer Control. 2013;20:97–110.
Welsh J, Palmer MB, Ajani JA, Liao Z, Swisher SG, Hofstetter WL, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118:2632–40.
Wolff C, Schott C, Porschewski P, Reischauer B, Becker KF. Successful protein extraction from over-fixed and long-term stored formalin-fixed tissues. PLoS ONE. 2011;6:e16353.
Chen Y, Lu Y, Wang Y, Yang H, Xia Y, Chen M, et al. Comparison of salvage chemoradiation versus salvage surgery for recurrent esophageal squamous cell carcinoma after definitive radiochemotherapy or radiotherapy alone. Dis Esophagus. 2014;27:134–40.
Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–74.
Murphy PR, Sato Y, Knee R. Phosphorothioate antisense oligonucleotides against basic fibroblast growth factor inhibit anchorage-dependent and anchorage independent growth of a malignant glioblastoma cell line. Mol Endocrinol. 1992;6:877–84.
Carmo CR, Lyons-Lewis J, Seckl MJ, Costa-Pereira AP. A novel requirement for Janus kinases as mediators of drug resistance induced by fibroblast growth factor-2 in human cancer cells. PLoS ONE. 2011;6:e19861.
Miyake H, Hara I, Gohji K, Yoshimura K, Arakawa S, Kamidono S. Expression of basic fibroblast growth factor is associated with resistance to cisplatin in a human bladder cancer cell line. Cancer Lett. 1998;30:121–6.
Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–9.
Kozin SV, Duda DG, Munn LL, Jain RK. Neovascularization after irradiation: what is the source of newly formed vessels in recurring tumors? J Natl Cancer Inst. 2012;104:899–905.
Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–15.
Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Take- masa I, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–90.
Acknowledgments
This work was supported by a grant-in-aid from the National Natural Science Foundation of China (No: U1204816) and Henan Provincial Science and Technology Bureau.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Li, X., Yang, H. et al. Expression of basic fibroblast growth factor, CD31, and α-smooth muscle actin and esophageal cancer recurrence after definitive chemoradiation. Tumor Biol. 35, 7275–7282 (2014). https://doi.org/10.1007/s13277-014-1987-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1987-9