Abstract
Proton pump inhibitors (PPIs) are widely prescribed as first-line therapy for the treatment of acid-related diseases, such as peptic ulcers and gastro-esophageal reflux disease, and for the eradication of Helicobacter pylori. However, the therapeutic efficacy of conventional PPIs is considered limited because: (1) they are unstable under acidic conditions and require an enteric-coated formulation in clinical use; (2) they show high interindividual variability in pharmacokinetics due to genetic polymorphisms of cytochrome P450 (CYP) 2C19 metabolism; (3) they have a relatively slow onset of pharmacological action and may require several doses to achieve optimal acid suppression and symptom relief; and (4) they often do not provide stable suppression of gastric acid secretion over 24 h. Vonoprazan fumarate (TAK-438, hereinafter referred to as “vonoprazan”) is a new potassium-competitive acid blocker (P-CAB) developed to resolve the above limitations of conventional PPIs. Various physicochemical data have shown that vonoprazan has a high solubility and stability over a broad pH range in aqueous conditions. In addition, vonoprazan has a more potent and longer-lasting acid suppression effect than the conventional PPI, lansoprazole. Preclinical pharmacokinetic studies have shown that vonoprazan is accumulated and retained in the stomach for more than 24 h, even after it is eliminated from the plasma. From these findings, we propose that vonoprazan, which possesses a novel mode of action, can improve on the outcomes seen with conventional PPI-based treatments for acid-related diseases.
Funding
This review project, including the publication of this article, was funded by Takeda Pharmaceutical Company Limited.










Similar content being viewed by others
References
Huang JQ, Hunt RH. pH, healing rate and symptomatic relief in acid-related diseases. Yale J Biol Med. 1996;69:159–74.
Hirschowitz BI, Keeling D, Lewin M, et al. Pharmacological aspects of acid secretion. Dig Dis Sci. 1995;40(2 Suppl):3S–23S.
Urushidani T, Nagao T. Calyculin A, a phosphoprotein phosphatase inhibitor, stimulates acid secretion in isolated gastric glands. Am J Physiol. 1996;270(1 Pt 1):G103–12.
Forte JG, Ganser A, Beesley R, Forte TM. Unique enzymes of purified microsomes from pig fundic mucosa. K+-stimulated adenosine triphosphatase and K+-stimulated pNPPase. Gastroenterology. 1975;69:175–89.
Chow DC, Forte JG. Functional significance of the beta-subunit for heterodimeric P-type ATPases. J Exp Biol. 1995;198:1–17.
Rabon E, Cuppoletti J, Malinowska D, et al. Proton secretion by the gastric parietal cell. J Exp Biol. 1983;106:119–33.
Herrmann M, Selige J, Raffael S, Sachs G, Brambilla A, Klein T. Systematic expression profiling of the gastric H+/K+ ATPase in human tissue. Scand J Gastroenterol. 2007;42:1275–88.
Kraut JA, Helander KG, Helander HF, Iroezi ND, Marcus EA, Sachs G. Detection and localization of H+-K+-ATPase isoforms in human kidney. Am J Physiol Renal Physiol. 2001;281(4):F763–8.
Forte JG, Hanzel DK, Okamoto C, Chow D, Urushidani T. Membrane and protein recycling associated with gastric HCl secretion. J Intern Med Suppl. 1990;732:17–26.
Howden CW, Burget DW, Hunt RH. Appropriate acid suppression for optimal healing of duodenal ulcer and gastro-oesophageal reflux disease. Scand J Gastroenterol Suppl. 1994;201:79–82.
Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51(Suppl 1):59–67.
Hunt RH. Importance of pH control in the management of GERD. Arch Intern Med. 1999;159:649–57.
Mössner J, Caca K. Developments in the inhibition of gastric acid secretion. Eur J Clin Invest. 2005;35:469–75.
Sachs G, Meyer-Rosberg K, Scott DR, Melchers K. Acid, protons and Helicobacter pylori. Yale J Biol Med. 1996;69:301–16.
Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of H. pylori infections: 2009 revised edition. Helicobacter. 2010;15:1–20.
Yuan Y, Wang CC, Yuan YH, et al. The proportion of patients who are free of reflux symptoms during the initial days of treatment with proton pump inhibitors (PPIs) in GERD trials: a meta-analysis. Gastroenterology. 2008;134(Suppl 1):A174.
Hunt RH, Scarpignato C. Potassium-competitive acid blockers (P-CABs): are they finally ready for prime time in acid-related disease? Clin Trans Gastroenterol. 2015;6:e119. doi:10.1038/ctg.2015.39.
Scarpignato C, Hunt RH. Proton pump inhibitors: the beginning of the end or the end of the beginning? Curr Opin Pharmacol. 2008;8(6):677–84.
Tytgat GN. Are there unmet needs in acid suppression? Best Pract Res Clin Gastroenterol. 2004;18(Suppl):67–72.
Fass R, Shapiro M, Dekel R, Sewell J. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease - where next? Aliment Pharmacol Ther. 2005;22(2):79–94.
Japanese Society for Gastroenterology. Guidelines for the management of GERD (2009). http://minds4.jcqhc.or.jp/minds/GERD/gerd_gl.pdf.
Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374–84.
Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53.
Ashida K. Notes on features and uses of medications used for PPI-resistant GERD, drugs under development and future prospects. Jpn J Med Pharm Sci. 2014;71:591–6.
Stewart B, Wallmark B, Sachs G. The interaction of H+ and K+ with the partial reactions of gastric (H++K+)-ATPase. J Biol Chem. 1981;256:2682–90.
Mendlein J, Sachs G. Interaction of a K+ -competitive inhibitor, a substituted imidazo[1,2a]pyridine, with the phospho- and dephosphoenzyme forms of H+, K+-ATPase. J Biol Chem. 1990;265:5030–6.
Parsons ME, Keeling DJ. Novel approaches to the pharmacological blockade of gastric acid secretion. Expert Opin Investig Drugs. 2005;14:411–21.
Kahrilas PJ, Dent J, Lauritsen K, et al. A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol. 2007;5:1385–91.
Berg AL, Böttcher G, Andersson K, et al. Early stellate cell activation and veno-occlusive-disease (VOD)-like hepatotoxicity in dogs treated with AR-H047108, an imidazopyridine proton pump inhibitor. Toxicol Pathol. 2008;36:727–37.
Dent J, Kahrilas PJ, Hatlebakk J, et al. A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol. 2008;103:20–6.
Kondo M, Kawamoto M, Hasuoka A, et al. High-throughput screening of potassium-competitive acid blockers. J Biomol Screen. 2012;17:177–82.
Nishida H, Hasuoka A, Arikawa Y, et al. Discovery, synthesis, and biological evaluation of novel pyrrole derivatives as highly selective potassium-competitive acid blockers. Bioorg Med Chem. 2012;20:3925–38.
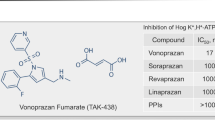
Arikawa Y, Nishida H, Kurasawa O, et al. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanaminefumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J Med Chem. 2012;55:4446–56.
Takeda Takecab (vonoprazan tablets). Japanese Common Technical Document. Available at: http://www.pmda.go.jp/drugs/2014/P201400173/index.html. Accessed 20 Apr 2016.
Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther. 2016;43(2):240–51.
Mizokami Y, Ashida K, Soen S, et al. TAK-438 versus lansoprazole 15 mg for secondary prevention of peptic ulcers associated with non-steroidal anti-inflammatory drug (NSAID) therapy: results of a phase 3 trial. Gastroenterology. 2014;146(5,Suppl 1):S-739.
Kawai T, Ashida K, Mizokami Y, et al. TAK-438 versus lansoprazole 15 mg for secondary prevention of peptic ulcers associated with low-dose aspirin therapy: results of a phase 3 trial. Gastroenterology. 2014;146(5,Suppl 1):S-739.
Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016. doi:10.1136/gutjnl-2015-311304 (Epub ahead of print).
Hori Y, Matsukawa J, Takeuchi T, et al. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther. 2011;337:797–804.
Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010;335:231–8.
Adachi K, Katsube T, Kawamura A, et al. CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment Pharmacol Ther. 2000;14(10):1259–66.
Furuta T, Ohashi K, Kosuge K, et al. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther. 1999;65:552–61.
Shirai N, Furuta T, Xiao F, et al. Comparison of lansoprazole and famotidine for gastric acid inhibition during the daytime and night-time in different CYP2C19 genotype groups. Aliment Pharmacol Ther. 2002;16(4):837–46.
Yamada S, Onda M, Kato S, et al. Genetic differences in CYP2C19 single nucleotide polymorphisms among four Asian populations. J Gastroenterol. 2001;36:669–72.
Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007;81:521–28.
Furuta T, Shirai N, Watanabe F, et al. Effect of cytochrome P4502C19 genotypic differences on cure rates for gastroesophageal reflux disease by lansoprazole. Clin Pharmacol Ther. 2002;72:453–60.
Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprasole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther. 2016;43(10):1048–59.
Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol. 2015;25(6):e94.
Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41(7):636–48.
Nagaya H, Satoh H, Kubo K, Maki Y. Possible mechanism for the inhibition of gastric (H++K+)-adenosine triphosphatase by the proton pump inhibitor AG-1749. J Pharmacol Exp Ther. 1989;248:799–805.
Fock KM, Ang TL, Bee LC, Lee EJ. Proton pump inhibitors: do differences in pharmacokinetics translate into differences in clinical outcomes? Clin Pharmacokinet. 2008;47:1–6.
Gedda K, Scott D, Besancon M, Lorentzon P, Sachs G. Turnover of the gastric H+, K+-adenosine triphosphatase α subunit and its effect on inhibition of rat gastric acid secretion. Gastroenterology. 1995;109:1134–41.
Sachs G. Improving on PPI-based therapy of GORD. Eur J Gastroenterol Hepatol. 2001;13(Suppl 1):S35–41.
Bytzer P, Blum AL. Personal view: rationale and proposed algorithms for symptom-based proton pump inhibitor therapy for gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20(4):389–98.
Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther. 2006;23(Suppl 2):2–8.
Tytgat GN. Shortcomings of the first-generation proton pump inhibitors. Eur J Gastroenterol Hepatol. 2001;13(Suppl 1):S29–33.
Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H, K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther. 2011;339:412–20.
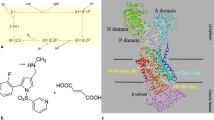
Abe K, Tani K, Fujiyoshi Y. Conformational rearrangement of gastric H+, K+-ATPase induced by an acid suppressant. Nat Commun. 2011;2:155–61.
Matsukawa J, Hori Y, Nishida H, kajino M, Inatomi N. A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol. 2011;81:1145–51.
Takeda Takecab (vonoprazan tablets) package insert. Available at: http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/400256_2329030F1020_1_04. Accessed 20 Apr 2016.
Hatlebakk JG, Katz PO, Camacho-Lobato L, Castell DO. Proton pump inhibitors: better acid suppression when taken before a meal than without a meal. Aliment Pharmacol Ther. 2000;14(10):1267–72.
Wada F, Murase K, Isomoto H, et al. Polymorphism of CYP2C19 and gastric emptying in patients with proton pump inhibitor-resistant gastric ulcers. J Int Med Res. 2002;30:413–21.
Klotz U. Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem. Int J Clin Pharmacol Ther. 2006;44:297–302.
Andersson K, Carlsson E. Potassium-competitive acid blockade: a new therapeutic strategy in acid-related diseases. Pharmacol Ther. 2005;108:294–307.
Zhou B, Huang Y, Li H, et al. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016;27:339–47.
Cunningham R, Dial S. Is over-use of proton pump inhibitors fuelling the current epidemic of Clostridium difficle-associated diarrhea? J Hosp Infect. 2008;70:1–6.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Takeda Pharmaceutical Company Limited.
We express our thanks to Mr. Masayuki Aboshi and Mr. Shigeki Okita of the Medical Science Liaison Group, Medical Affairs Department, Japan Research Center, Takeda Pharmaceutical Company Limited, and Mr. Francois Jones of Quintiles Transnational Japan Co., Ltd. for their support in drafting this article. We also thank the Medical Science Liaison Group, Medical Affairs Department, Japan Research Center, Takeda Pharmaceutical Company Limited for providing scientific advice. Dr. Keisuke Ishida of SunFlare Co., Ltd. provided medical writing support, which was funded by Takeda Pharmaceutical Company Limited. Mr. Yoshitaka Sato of Saikou, provided medical illustration service, which was funded by Takeda Pharmaceutical Company Limited.
This article was originally published in Japanese in the journal Progress in Medicine. Permission to translate and republish the article in English was granted by the publisher of Progress in Medicine. Kazuyoshi Otake, Yuichi Sakurai, Haruyuki Nishida, Hideo Fukui, Yoshihiko Tagawa, Hitomi Yamasaki, Masatoshi Karashima, Keiichi Otsuka, Nobuhiro Inatomi. Vonoprazan Fumarate (TAK-438). A New Potassium-Competitive Acid Blocker (P-CAB) with a Novel Mode of Action—physicochemical Properties, Non-Clinical Data, and a Proposed Mode of Action. Progress in Medicine 2014:34(12):2183–2194. Translation of the article from Japanese to English was funded by Takeda Pharmaceuticals USA, Inc. and performed by a native Japanese-speaking translator specialized in the life sciences. The translation was conducted according to rigid ISO 9001:2008 and EN 15038:2006 certified quality assurance processes. This article is a translation of the original Japanese article to English with updates.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Kazuyoshi Otake, Yuuichi Sakurai, Haruyuki Nishida, Hideo Fukui, Yoshihiko Tagawa, Hitomi Yamasaki, Masatoshi Karashima, Keiichi Otsuka and Nobuhiro Inatomi are employees of Takeda Pharmaceutical Company Limited, and there are no other applicable matters regarding conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/92D4F06050CF12A4.
Rights and permissions
About this article
Cite this article
Otake, K., Sakurai, Y., Nishida, H. et al. Characteristics of the Novel Potassium-Competitive Acid Blocker Vonoprazan Fumarate (TAK-438). Adv Ther 33, 1140–1157 (2016). https://doi.org/10.1007/s12325-016-0345-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0345-2