Abstract
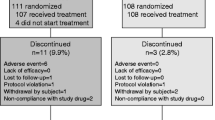
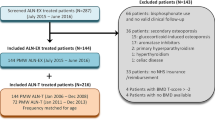
Alendronate is one of the most potent anti-osteoporotic agents for postmenopausal osteoporosis. However, high doses of alendronate cause esophageal irritation, myalgia, gastrointestinal discomfort and decrease of serum calcium level. Recently, Maxmarvil® was developed as an enteric-coated tablet containing alendronate (5 mg) and calcitriol (0.5 μg) to minimize these side effects of alendronate. In the present study, we evaluated the pharmacokinetic profile and examined the incidence of unfavorable effects after oral administration of Maxmarvil® in Korean healthy postmenopausal women without a previous history of fracture. In the in vitro dissolution test, alendronate was not released from Maxmarvil® in pH 1.2 phosphate buffer solution but released in pH 6.0 and 6.8 phosphate buffer solutions and completely dissolved in 30 min. After oral administration of Maxmarvil®, three out of 18 (16.7 %) women showed mild adverse effects; two myalgia and one upper gastrointestinal discomfort without heartburn. Most of these complaints disappeared during the study without additional treatment. The peak (U max) and the average (U ave) urinary excretion rate of alendronate and the time to reach U max (T max) were 2.94 μg/h, 0.901 μg/h and 6.77 h, respectively. The total cumulative urinary excretion of alendronate (Ae0–24 h) was 21.6 μg (0.432 % of oral alendornate), which was similar to the reported values. Taken together, enteric-coated Maxmarvil® is less harmful for the esophagus and gastrointestinal mucosa, shows the same pharmacokinetic profile to conventional alendronate (70 mg) and improves the tolerability of medication in clinical practice.


Similar content being viewed by others
References
Byun, D., and J. Mok. 2013. Endoscopic comparison of enterocoating alendronate with calcitriol combined drug and alendronate in Korean postmenopausal women. The Korean Journal of Internal Medicine (in press).
Cummings, S.R., and L.J. Melton. 2002. Epidemiology and outcomes of osteoporotic fractures. Lancet 359: 1761–1767.
Gertz, B.J., S.D. Holland, W.F. Kline, B.K. Matuszewski, and A.G. Porras. 1993. Clinical pharmacology of alendronate sodium. Osteoporosis International 3: S13–S16.
Gertz, B.J., S.D. Holland, W.F. Kline, B.K. Matuszewski, A. Freeman, H. Quan, K.C. Lasseter, J.C. Mucklow, and A.G. Porras. 1995. Studies of the oral bioavailability of alendronate. Clinical Pharmacology and Therapeutics 58: 288–298.
Han, H.-K., H.-J. Shin, and D.H. Ha. 2012. Improved oral bioavailability of alendronate via the mucoadhesive liposomal delivery system. European Journal of Pharmaceutical Sciences 46: 500–507.
Iwamoto, J., T. Takeda, Y. Sato, and M. Uzawa. 2005. Early changes in urinary cross-linked N-terminal telopeptides of type I collagen level correlate with 1-year response of lumbar bone mineral density to alendronate in postmenopausal Japanese women with osteoporosis. Journal of Bone and Mineral Metabolism 23: 238–422.
Kushida, K., M. Shiraki, T. Nakamura, H. Kishimoto, H. Morii, K. Yamamoto, K. Kaneda, M. Fukunaga, T. Inoue, M. Nakashima, and H. Orimo. 2004. Alendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: A 3-year follow-up study. Journal of Bone and Mineral Metabolism 22: 462–468.
Lee, Y.-R., S.K. Jung, S.K. Yang, K.H. Choi, Y.C. Shin, H.G. Jeon, S. Kang, and H.J. Lee. 2006. Bioequivalence of Daewoong alendronate tablet to Fosamax table (sodium alendronate 70 mg). Journal of Korean Pharmaceutical Sciences 36: 137–142.
Lin, J.H. 1996. Bisphosphonates: A review of their pharmacokinetic properties. Bone 18: 75–85.
Machin, D., M. Campbell, P. Fayers, and A. Pinol. 1997. Sample size tables for clinical studies, 2nd ed. Malden, MA: Blackwell Science.
Monk, R.D., and D.A. Bushinsky. 2003. Kidney stones. In Williams textbook of endocrinology, ed. P.R. Larsen, H.M. Kronenberg, S. Melmed, and K.S. Polonsky, 1411–1425. Philadelphia: WB Saunders.
Naruse, S., A. Hisaka, H. Watanabe, T. Taniguchi, Y. Kato, K. Tani, and H. Arizono. 2004. Pharmacokinetic study of alendronate in postmenopausal Japanese women. Rinsho Iyaku 20: 1227–1234.
Ptacek, P., J. Klima, and J. Macek. 2002. Determination of aldendronate in human urine as 9-fluorenylmethyl derivative by high-performance liquid chromatography. Journal of Chromatography B 768: 111–116.
Porras, A.G., S.D. Holland, and B.J. Gertz. 1999. Pharmacokinetics of alendronate. Clinical Pharmacokinetics 36: 315–328.
Rhee, Y., M. Kang, Y. Min, D. Byun, Y. Chung, C. Ahn, K. Baek, J. Mok, D. Kim, D. Kim, H. Kim, Y. Kim, S. Myoung, D. Kim, and S.-K. Lim. 2006. Effects of a combined alendronate and calcitriol agent (Maxmarvil®) on bone metabolism in Korean postmenopausal women: A multicenter, double-blind, randomized, placebo-controlled study. Osteoporosis International 17: 1801–1807.
Sato, M., W. Grasser, N. Endo, R. Akins, H. Simmons, D.D. Thompson, E. Golub, and G.A. Rodan. 1991. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. Journal of Clinical Investigation 88: 2095–2105.
Shiraki, M., K. Kushida, M. Fukunaga, H. Kishimoto, K. Kaneda, H. Minaguchi, T. Inoue, A. Tomita, Y. Nagata, M. Nakashima, and H. Orimo. 1998. A placebo-controlled, single-blind study to determine the appropriate alendronate dosage in postmenopausal Japanese patients with osteoporosis. Endocrine Journal 45: 191–201.
Shiraki, M., K. Kushida, M. Fukunaga, H. Kishimoto, M. Taga, T. Nakamura, K. Kaneda, H. Minaguchi, T. Inoue, H. Morii, A. Tomita, K. Yamamoto, Y. Nagata, M. Nakashima, and H. Orimo. 1999. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The Alendronate Phase III Osteoporosis Treatment Research Group. Osteoporosis International 10: 183–192.
Sparidans, R.W., I.M. Twiss, and S. Talbot. 1998. Bisphosphonates in bone diseases. Pharmacy World & Science 20: 206–213.
Watts, N.B., and D.L. Diab. 2010. Long-term use of bisphosphonates in osteoporosis. Journal of Clinical Endocrinology and Metabolism 95: 1555–1565.
Zar, J.H. 1984. Biostatistical analysis, 2nd ed. Englewood Cliffs: Prentice-Hall.
Acknowledgments
This research was supported by the Yuyu Pharma, Inc. with study approved as AJIRB-MED-CT1-10-335.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, YS., Choi, Y.J. & Kim, S.H. Improved dosage form of the combined alendronate and calcitriol (Maxmarvil®) on the absorption of alendronate in Korean postmenopausal women. Arch. Pharm. Res. 36, 966–972 (2013). https://doi.org/10.1007/s12272-013-0124-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0124-4