Abstract
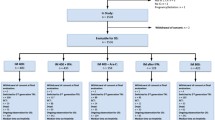
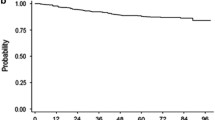
The TOPS trial evaluated high- (800 mg/day; n = 319) versus standard-dose (400 mg/day; n = 157) imatinib in patients newly diagnosed with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase. Patients had a minimum follow-up of 42 months or discontinued early. Major molecular response (MMR) rates were similar between arms at (51.6 vs 50.2 % for 400 and 800 mg/day, respectively; P = 0.77) and by (75.8 vs 79.0 %; P = 0.4807) 42 months. There were no differences in event-free survival (EFS), progression-free survival (PFS), or overall survival (OS) between arms. The estimated rates of PFS on treatment and OS at 42 months were significantly higher in patients with MMR at 6, 12, and 18 months compared with those without MMR. Adverse events were more frequent with high-dose imatinib. Patients with ≤1 treatment interruption (vs >1) and those able to maintain imatinib ≥600 mg/day (vs <600 mg/day) in the first year of treatment had faster and higher response rates, but no improvement in EFS or PFS. Adherence to prescribed dose without interruption may be more important than initiation of therapy with higher doses of imatinib. Achievement of MMR correlated with long-term clinical outcomes.



Similar content being viewed by others
References
Cortes JE, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim DW, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28:424–30.
Branford S, Fletcher L, Cross NC, Muller MC, Hochhaus A, Kim DW, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112:3330–8.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51.
Cortes JE, Kantarjian HM, Goldberg SL, Powell BL, Giles FJ, Wetzler M, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27:4754–9.
Preudhomme C, Guilhot J, Nicolini FE, Guerci-Bresler A, Rigal-Huguet F, Maloisel F, et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010;363:2511–21.
Baccarani M, Rosti G, Castagnetti F, Haznedaroglu I, Porkka K, Abruzzese E, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet study. Blood. 2009;113:4497–504.
Castagnetti F, Palandri F, Amabile M, Testoni N, Luatti S, Soverini S, et al. Results of high-dose imatinib mesylate in intermediate Sokal risk chronic myeloid leukemia patients in early chronic phase: a phase 2 trial of the GIMEMA CML working party. Blood. 2009;113:3428–34.
Hughes TP, Branford S, White DL, Reynolds J, Koelmeyer R, Seymour JF, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic-phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008;112:3965–73.
O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
Yeung DT, Osborn MP, White DL, Branford S, Kornhauser M, Slader C, et al. Early switch to nilotinib does not overcome the adverse outcome for CML patients failing to achieve early molecular response on imatinib, despite excellent overall outcomes in the TIDEL-II trial. Blood (ASH annual meeting abstracts). 2012;(abstract 3771).
Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-{alpha} in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29:1634–42.
Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–23.
Noens L, van Lierde MA, De Bock R, Verhoef G, Zachee P, Berneman Z, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401–11.
Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–8.
Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–32.
Hughes TP, Hochhaus A, Branford S, Muller MC, Kaeda JS, Foroni L, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the international randomized study of interferon versus STI571 (IRIS). Blood. 2010;116:3758–65.
Press RD, Love Z, Tronnes AA, Yang R, Tran T, Mongoue-Tchokote S, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107:4250–6.
Kantarjian H, O’Brien S, Shan J, Huang X, Garcia-Manero G, Faderl S, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia: need for new response definitions? Cancer. 2008;112:837–45.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84.
National Comprehensive Cancer Network. Chronic myeloid leukemia. v3. 2014.
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9.
Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–51.
Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–203.
Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood 2014;123:1353–60.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–70.
Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119:1123–9.
Acknowledgments
This work was supported by research funding from Novartis Pharmaceuticals Corporation. We thank Erinn Goldman, Ph.D. (ArticulateScience, LLC), for medical editorial assistance with this manuscript. We would also like to thank all investigators and patients who participated in the TOPS trial.
Conflict of interest
Dr. Baccarani acted as a consultant for Novartis and Bristol-Myers Squibb Company, Pfizer, and Ariad. Dr. Druker is a principal investigator on Novartis and Bristol-Myers Squibb clinical trials. His institution receives payment from these companies to cover patient costs, nurse/data manager salaries, and institutional overhead. He does not receive salary, and his laboratory does not receive funds. Dr. Branford received honoraria from Novartis and Bristol-Myers Squibb. Dr. Kim received research funding, honoraria and acted as a consultant for Novartis, Wyeth, and Bristol-Myers Squibb, received honoraria for Ilyang, and is a clinical trial lead for Pfizer. Dr. Pane has nothing to disclose. Dr. Mongay, Dr. Mone, and Christine-Elke Ortmann are Novartis employees and Dr. Mone holds Novartis stock. Dr. Kantarjian has nothing to disclose. Dr. Hughes received research funding/honoraria from Novartis and Bristol-Myers Squibb and consultancy from Ariad. Dr. Cortes received research funding and acted as a consultant for Bristol-Myers Squibb, Novartis, BMS, and Pfizer. Dr. Guilhot received research funding/honoraria from Novartis.
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Baccarani, M., Druker, B.J., Branford, S. et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol 99, 616–624 (2014). https://doi.org/10.1007/s12185-014-1566-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-014-1566-2