Abstract
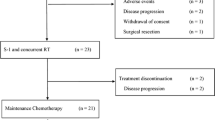
There has been a pressing need to develop optimal regimen for neoadjuvant chemotherapy (NAC) for pancreatic cancer (PC). The safety and efficacy of gemcitabine, S-1, and LV combination (GSL) therapy as NAC for borderline resectable (BR) and locally advanced (LA) PC was evaluated in this phase II study. Patients with pathologically proven BR or LA PC were enrolled and gemcitabine 1000 mg/m2 by 30-min infusion on day 1, S-1 40 mg/m2 orally twice daily, and LV 25 mg orally twice daily on days 1–7 every 2 weeks were provided, and evaluation by CT every 2 courses was performed. The primary end point was R0 resection rate, and the secondary endpoints were resection rate, response rate, adverse events, surgical outcomes, and survival. Twenty-four patients with PC (21 BR and 3 LA) were enrolled. Response rate and disease control rate of NAC were 17.4 and 87.0%. Grade 3 and 4 toxicities involved neutropenia (34.8%), anorexia (17.4%), and mucositis (17.4%). Serum CA19-9 level decreased by 52.2%. Resection rate was 60.9% after the median of 4 cycles and R0 resection rate was 76.5% in patients undergoing laparotomy. NAC-GSL is a feasible treatment option for BR and LAPC.



Similar content being viewed by others
References
Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211(4):447 – 58.
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13.
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. https://doi.org/10.1200/JCO.2006.07.9525.
Okusaka T, Funakoshi A, Furuse J, Boku N, Yamao K, Ohkawa S, et al. A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2008;61(4):615 – 21. https://doi.org/10.1007/s00280-007-0514-8.
Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Toda N, et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer. 2012;106(12):1934–9. https://doi.org/10.1038/bjc.2012.183.
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31(13):1640–8. https://doi.org/10.1200/JCO.2012.43.3680.
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastroint Surg. 2000;4(6):567 – 79.
Network NCC. NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Version 2. 2017. http://www.nccnorg/professionals/physician_gls/pdf/pancreaticpdf.
Nakai Y, Isayama H, Saito K, Sasaki T, Takahara N, Hamada T, et al. A phase I trial of gemcitabine, S-1 and LV combination (GSL) therapy in advanced pancreatic cancer. Cancer Chemother Pharmacol. 2014;74(5):911–5. https://doi.org/10.1007/s00280-014-2563-0.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228 – 47. https://doi.org/10.1016/j.ejca.2008.10.026.
National Cancer Institute USDOHAHS. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. https://www.evsncinihgov/ftp1/CTCAE/CTCAE_403_2010-06-14_QuickReference_5x7pdf. 2009.
Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127(11):1335–9.
Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118(23):5749–56. https://doi.org/10.1002/cncr.27636.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. https://doi.org/10.1056/NEJMoa1011923.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691 – 703. https://doi.org/10.1056/NEJMoa1304369.
Okusaka T, Ikeda M, Fukutomi A, Ioka T, Furuse J, Ohkawa S, et al. Phase II study of FOLFIRINOX for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014;105(10):1321–6. https://doi.org/10.1111/cas.12501.
Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Omuro Y, et al. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77(3):595–603. https://doi.org/10.1007/s00280-016-2972-3.
Kim SS, Nakakura EK, Wang ZJ, Kim GE, Corvera CU, Harris HW, et al. Preoperative FOLFIRINOX for borderline resectable pancreatic cancer: Is radiation necessary in the modern era of chemotherapy? J Surg Oncol. 2016;114(5):587 – 96. https://doi.org/10.1002/jso.24375.
de WMR, Talamonti, Baker MS, Posner MS, Roggin M, Matthews K. J et al. Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: A pilot study. J Surg Oncol. 2018;117(3):354 – 62. https://doi.org/10.1002/jso.24872.
Hamada C, Okusaka T, Ikari T, Isayama H, Furuse J, Ishii H, et al. Efficacy and safety of gemcitabine plus S-1 in pancreatic cancer: a pooled analysis of individual patient data. British journal of cancer. 2017. https://doi.org/10.1038/bjc.2017.128.
Koizumi W, Boku N, Yamaguchi K, Miyata Y, Sawaki A, Kato T, et al. Phase II study of S-1 plus leucovorin in patients with metastatic colorectal cancer. Ann Oncol. 2010;21(4):766 – 71. https://doi.org/10.1093/annonc/mdp371.
Hironaka S, Sugimoto N, Yamaguchi K, Moriwaki T, Komatsu Y, Nishina T, et al. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):99–108. https://doi.org/10.1016/s1470-2045(15)00410-6.
Ueno M, Okusaka T, Omuro Y, Isayama H, Fukutomi A, Ikeda M, et al. A randomized phase II study of S-1 plus oral leucovorin versus S-1 monotherapy in patients with gemcitabine-refractory advanced pancreatic cancer. Ann Oncol. 2016;27(3):502–8. https://doi.org/10.1093/annonc/mdv603.
Zhan HX, Xu JW, Wu D, Wu ZY, Wang L, Hu SY, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med. 2017;6(6):1201–19. https://doi.org/10.1002/cam4.1071.
Mellon EA, Jin WH, Frakes JM, Centeno BA, Strom TJ, Springett GM, et al. Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy. Acta Oncol. 2016. https://doi.org/10.1080/0284186X.2016.1256497.
Zhao Q, Rashid A, Gong Y, Katz MH, Lee JE, Wolf R, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. 2012;16(1):29–37. https://doi.org/10.1016/j.anndiagpath.2011.08.005.
Chatterjee D, Katz MH, Rashid A, Varadhachary GR, Wolff RA, Wang H, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118(12):3182–90. https://doi.org/10.1002/cncr.26651.
Satoi S, Yamaue H, Kato K, Takahashi S, Hirono S, Takeda S, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepato Biliary Pancr Sci. 2013. https://doi.org/10.1007/s00534-013-0616-0.
Boone BA, Steve J, Zenati MS, Hogg ME, Singhi AD, Bartlett DL, et al. Serum CA 19 – 9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21(13):4351–8. https://doi.org/10.1245/s10434-014-3842-z.
Nakai Y, Kawabe T, Isayama H, Sasaki T, Yagioka H, Yashima Y, et al. CA 19 – 9 response as an early indicator of the effectiveness of gemcitabine in patients with advanced pancreatic cancer. Oncology. 2008;75(1–2):120–6. https://doi.org/10.1159/000155213.
Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, et al. CA 19 – 9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9(2):132–8. https://doi.org/10.1016/s1470-2045(08)70001-9.
Takahashi H, Ohigashi H, Ishikawa O, Eguchi H, Gotoh K, Yamada T, et al. Serum CA19-9 alterations during preoperative gemcitabine-based chemoradiation therapy for resectable invasive ductal carcinoma of the pancreas as an indicator for therapeutic selection and survival. Ann Surg. 2010;251(3):461–9. https://doi.org/10.1097/SLA.0b013e3181cc90a3.
Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N, et al. Prognostic impact of normalization of serum tumor markers following neoadjuvant chemotherapy in patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother Pharmacol. 2017;79(4):801 – 11. https://doi.org/10.1007/s00280-017-3281-1.
Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–24. https://doi.org/10.1016/s0140-6736(16)32409-6.
Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248 – 57. https://doi.org/10.1016/s0140-6736(16)30583-9.
Acknowledgements
The authors would like to thank Drs. Saburo Matsubara, Natsuyo Yamamoto, Kenji Hirano for their patient management.
Funding
This research was supported by Japanese foundation for multidisciplinary treatment of cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hiroyuki Isayama and Yousuke Nakai received research funding from Taiho Pharmaceutical Co. All remaining authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Hilsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saito, K., Isayama, H., Sakamoto, Y. et al. A phase II trial of gemcitabine, S-1 and LV combination (GSL) neoadjuvant chemotherapy for patients with borderline resectable and locally advanced pancreatic cancer. Med Oncol 35, 100 (2018). https://doi.org/10.1007/s12032-018-1158-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1158-8