Abstract
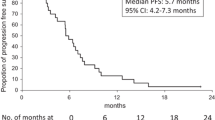
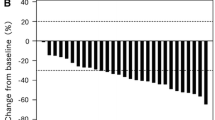
The efficacy of bevacizumab combined with infusional 5-fluorouracil/leucovorin (5-FU/LV) plus irinotecan (FOLFIRI) as the second-line treatment for metastatic colorectal cancer (mCRC) has not been fully clarified, although bevacizumab combined with infusional 5-FU/LV plus oxaliplatin (FOLFOX) in the second-line setting has demonstrated a survival benefit. We investigated the efficacy of bevacizumab plus FOLFIRI in mCRC patients who failed oxaliplatin-containing regimens without bevacizumab. Patients who received bevacizumab plus FOLFIRI or bevacizumab plus FOLFOX as second-line chemotherapy between July 2007 and March 2008 were registered (trial registration: UMIN000001547). Patient background data and progression-free survival (PFS), overall survival (OS), response, and bevacizumab-related adverse events were prospectively collected every 6 months. A total of 195 patients were enrolled from 26 institutions. Among them, 115 patients received bevacizumab plus FOLFIRI after failure of oxaliplatin and fluoropyrimidine (FOLFIRI+BV after OX/FU group), and 45 patients received bevacizumab plus FOLFOX after failure of irinotecan and fluoropyrimidine (FOLFOX+BV after IRI/FU group). Median PFS was 8.3 months (95% confidence interval [CI], 6.7–9.9) for the FOLFIRI+BV after OX/FU group and 7.8 months (95% CI, 5.8–9.7) for the FOLFOX+BV after IRI/FU group. Median OS was 21.6 months (95% CI, 17.6–25.6) and 16.5 months (95% CI, 11.8–21.2), respectively. Overall response rates were 25 and 29%, respectively. The most common grade ≥3 bevacizumab-related adverse events were hypertension (5.0%) and bleeding (3.8%). FOLFIRI+BV after OX/FU showed comparable efficacy to FOLFOX+BV after IRI/FU.


Similar content being viewed by others
Abbreviations
- 5-FU/LV:
-
5-Flurouracil/leucovorin
- FOLFIRI:
-
Infusional 5-fluorouracil/leucovorin plus irinotecan
- FOLFOX:
-
Infusional 5-fluorouracil/leucovorin plus oxaliplatin
- mCRC:
-
Metastatic colorectal cancer
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- TCTG:
-
Tsukuba Cancer Clinical Trial Group
- ECOG:
-
Eastern Cooperative Oncology Group
- PS:
-
Performance status
- UMIN:
-
University Hospital Medical Information Network
- EGFR:
-
Epidermal growth factor receptor
- CI:
-
Confidence interval
- FOLFIRI+BV after OX/FU group:
-
Patients who received bevacizumab plus FOLFIRI after failure of oxaliplatin and fluoropyrimidine
- FOLFOX+BV after IRI/FU group:
-
Patients who received bevacizumab plus FOLFOX after failure of irinotecan and fluoropyrimidine
- FLOX:
-
Bolus 5-fluorouracil/leucovorin plus oxaliplatin
- XELOX:
-
Capecitabine plus oxaliplatin
References
Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37.
Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113–9.
Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol. 2007;25:4779–86.
Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44.
ETHICAL guidelines for epidemiological research. http://www.niph.go.jp/wadai/ekigakurinri/guidelines.pdf.
Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–204.
Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–71.
Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51.
Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13.
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65.
Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34.
Yildiz R, Buyukberber S, Uner A, et al. Bevacizumab plus irinotecan-based therapy in metastatic colorectal cancer patients previously treated with oxaliplatin-based regimens. Cancer Invest. 2010;28:33–7.
Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842–7.
Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist. 2009;14:862–70.
Bekaii-Saab TS, Bendell JC, Cohn AL, et al. Bevacizumab (BV) plus chemotherapy (CT) in second-line metastatic colorectal cancer (mCRC): Initial results from ARIES, a second BV observational cohort study (OCS). J Clin Oncol 2010;28:15s (suppl; abstr 3495).
Acknowledgments
The authors would like to thank all of the investigators at the 26 institutions and the patients who participated in this study. Study investigators: Chiba Cancer Center: T. Denda; Dongo Hospital: M. Matsuoka; Hokkaido University Hospital: Y. Komatsu, I. Iwanaga; Ibaraki Prefectural Central Hospital and Cancer Center: K. Amagai, M. Ozeki; Iwate prefectural Central Hospital: S. Kato; Kanagawa Cancer Center: S. Motomura, C. Hashimoto; Kinki University School of Medicine: T. Satoh, S. Fumita; Kitazato University East Hospital: W. Koizumi, T. Sasaki; Kobe University Hospital: T. Okuno, Y. Fujishima; Kushiro City General Hospital: T. Abe; Kyushu University Hospital: E. Baba; Minoh City Hospital: K. Kato, Y. Miyake; Mito Medical Center: T. Yamaguchi, S. Yoshida; Nagoya Memorial Hospital: K. Ina, R. Furuta; National Cancer Center Hospital: H. Yamada, A. Takashima; National Cancer Center Hospital East: T. Yoshino, H. Bando; National Kyushu Cancer Center: T. Esaki, M. Ohta; Osaka City General Hospital: S. Tokunaga, M. Hattori; Ritsurin Hospital: S. Indo, A. Teramoto; Saitama Medical University International Medical Center: K. Yamashita; Sakai Municipal Hospital: M. Fukunaga, H. Takemoto; Shikoku Cancer Center: T. Nishina, T. Kajiwara; Shizuoka Cancer Center: K. Yamazaki; Suita Municipal Hospital: K. Murata, S. Tanaka; Tokyo Medical University: K. Katsumata, Y. Mori; Tsukuba University Hospital: I. Hyodo, T. Moriwaki. This work was supported by the NPO Tsukuba Cancer Clinical Trial Group (TCTG). Portions of this study data have been previously presented at the 35th European Society for Medical Oncology Congress in 2010.
Conflict of interest
Dr. Hyodo received funds in an advisory role from Yakult Honsha and Chugai Pharmaceuticals. All other authors state that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moriwaki, T., Bando, H., Takashima, A. et al. Bevacizumab in combination with irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) in patients with metastatic colorectal cancer who were previously treated with oxaliplatin-containing regimens: a multicenter observational cohort study (TCTG 2nd-BV study). Med Oncol 29, 2842–2848 (2012). https://doi.org/10.1007/s12032-011-0151-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0151-2