Abstract
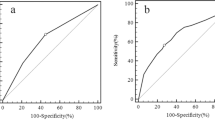
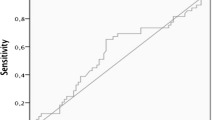
Recently, it has been suggested that thyrotropin (TSH) concentration can be used as a marker for prediction of thyroid malignancy. In this study, we aimed to investigate the association between TSH levels and prediction of malignancy in euthyroid patients with different Bethesda categories. The data of 1433 euthyroid patients with 3206 thyroid nodules who underwent thyroidectomy were screened retrospectively. The preoperative cytology results, thyroid function tests, thyroid autoantibodies, and presence of histopathological Hashimoto’s thyroiditis (HT) were recorded. Of the 1433 patients, 585 (40.8 %) had malignant and 848 (59.2 %) had benign histopathology. Malignant group had smaller nodule size, elevated TSH levels, and higher rate of presence of HT compared to benign group (p < 0.001, all). Cytology results of 3206 nodules were as follows: 832 nondiagnostic (ND), 1666 benign, 392 atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), 68 follicular neoplasm/suspicious for follicular neoplasm (FN/SFN), 133 suspicious for malignancy (SM), and 115 malignant. Both SM and malignant cytology groups had higher TSH levels than other 4 Bethesda categories (p < 0.05, all). Benign cytology group had significantly lower TSH levels compared to other cytology groups (p < 0.05, all). Patients with malignant final histopathology in ND and AUS/FLUS cytology groups had significantly higher TSH levels compared to patients with benign final histopathology (p < 0.05, all). Moreover, TSH levels showed to increase from Bethesda categories II to VI. In addition to cytology, higher TSH levels can be used as a supplementary marker in prediction of malignancy in certain Bethesda categories.



Similar content being viewed by others
References
E. Marqusee, C.B. Benson, M.C. Frates, P.M. Doubilet, P.R. Larsen, E.S. Cibas, S.J. Mandel, Usefulness of ultrasonography in the management of nodular thyroid disease. Ann. Intern. Med. 133(9), 696–700 (2000)
A. Belfiore, G.L. La Rosa, G.A. La Porta, D. Giuffrida, G. Milazzo, L. Lupo, C. Regalbuto, R. Vigneri, Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am. J. Med. 93(4), 363–369 (1992)
L. Davies, H.G. Welch, Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295(18), 2164–2167 (2006)
D. Danese, S. Sciacchitano, A. Farsetti, M. Andreoli, A. Pontecorvi, Diagnostic accuracy of conventional versus sonography-guided fineneedle aspiration biopsy of thyroid nodules. Thyroid 8(1), 15–21 (1998)
T. Yokozawa, A. Miyauchi, K. Kuma, M. Sugawara, Accurate and simple method of diagnosing thyroid nodules the modified technique of ultrasound-guided fine needle aspiration biopsy. Thyroid 5(2), 141–145 (1995)
L. Yassa, E.S. Cibas, C.B. Benson, M.C. Frates, P.M. Doubilet, A. Gawande, F.D. Moore Jr, B.W. Kim, V. Nosé, E. Marqusee, P.R. Larsen, E.K. Alexander, Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 111(6), 508–516 (2007)
A.L. Maia, L.S. Ward, G.A. Carvalho, H. Graf, R.M. Maciel, L.M. Maciel, P.W. Rosario, M. Vaisman, Thyroid nodules and differentiated thyroid cancer: Brazilian consensus. Arq. Bras. Endocrinol. Metab. 51(5), 867–893 (2007)
British Thyroid Association, Royal College of Physicians: British Thyroid Association Guidelines for the management of thyroid cancer, 2nd edn. (British Thyroid Association, London, 2007)
J.I. Lew, S.E. Rodgers, C.C. Solorzano, Developments in the use of ultrasound for thyroid cancer. Curr. Opin. Oncol. 22(1), 11–16 (2010)
L. Hegedus, Clinical practice. The thyroid nodule. N. Engl. J. Med. 351(17), 1764–1771 (2004)
G. Oler, J.M. Cerutti, High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer 115(5), 972–980 (2009)
L. Hegedus, S.J. Bonnema, F.N. Bennedbaek, Management of simple nodular goiter: current status and future perspectives. Endocr. Rev. 24(1), 102–132 (2003)
K. Boelaert, J. Horacek, R.L. Holder, J.C. Watkinson, M.C. Sheppard, J.A. Franklyn, Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J. Clin. Endocrinol. Metab. 91(11), 4295–4301 (2006)
E. Fiore, T. Rago, M.A. Provenzale, M. Scutari, C. Ugolini, F. Basolo, G. Di Coscio, P. Miccoli, L. Grasso, A. Pinchera, P. Vitti, L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr. Relat. Cancer 17(1), 231–239 (2010)
K. Gul, D. Ozdemir, A. Dirikoc, A. Oguz, D. Tuzun, H. Baser, R. Ersoy, B. Cakir, Are endogenously lower serum thyroid hormones new predictors for thyroid malignancy in addition to higher serum thyrotropin? Endocrine 37(2), 253–260 (2010)
M.R. Haymart, D.J. Repplinger, G.E. Leverson, D.F. Elson, R.S. Sippel, J.C. Jaume, H. Chen, Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J. Clin. Endocrinol. Metab. 93(3), 809–814 (2008)
E. Fiore, T. Rago, M.A. Provenzale, M. Scutari, C. Ugolini, F. Basolo, G. Di Coscio, P. Berti, L. Grasso, R. Elisei, A. Pinchera, P. Vitti, Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr. Relat. Cancer 16(4), 1251–1260 (2009)
E.S. Cibas, S.Z. Ali, NCI Thyoid FNA State of the Science Conference, The Bethesda System for reporting thyroid cytopathology. Am. J. Clin. Pathol. 132(5), 658–665 (2009)
Y.J. Choi, I. Jung, S.J. Min, H.J. Kim, J.H. Kim, S. Kim, J.S. Park, J.H. Shin, Y.M. Sohn, J.H. Yoon, J.Y. Kwak, Thyroid nodule with benign cytology: is clinical follow-up enough? PLoS One 8(5), e63834 (2013)
J.Y. Kwak, E.K. Kim, H.J. Kim, M.J. Kim, E.J. Son, H.J. Moon, How to combine ultrasound and cytological information in decision making about thyroid nodules. Eur. Radiol. 19(8), 1923–1931 (2009)
J.Y. Kwak, H. Koo, J.H. Youk, M.J. Kim, H.J. Moon, E.J. Son, E.K. Kim, Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology 254(1), 292–300 (2010)
J.H. Yoon, H.J. Moon, E.K. Kim, J.Y. Kwak, Inadequate cytology in thyroid nodules: should we repeat aspiration or follow-up? Ann. Surg. Oncol. 18(5), 1282–1289 (2011)
S.W. Lee, H.J. Lee, H.J. Kim, J. Lee, J.Y. Park, S.H. Kim, J. Kim, Combined categorical reporting systems of US and cytology findings for thyroid nodules: guidance on repeat fine-needle aspiration cytology. Radiology 266(3), 956–963 (2013)
V.Y. Jo, E.B. Stelow, S.M. Dustin, K.Z. Hanley, Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology. Am. J. Clin. Pathol. 134(3), 450–456 (2010)
C.Y. Eng, M.S. Quraishi, P.J. Bradley, Management of Thyroid nodules in adult patients. Head Neck Oncology 2, 11 (2010)
S.J. Mandel, A 64-year-old woman with a thyroid nodule. JAMA 292(21), 2632–2642 (2004)
M.R. Castro, R.P. Espiritu, R.S. Bahn, M.R. Henry, H. Gharib, P.J. Caraballo, J.C. Morris, Predictors of malignancy in patients with cytologically suspicious thyroid nodules. Thyroid 21(11), 1191–1198 (2011)
E.S. Kim, D.J. Lim, K.H. Baek, J.M. Lee, M.K. Kim, H.S. Kwon, K.H. Song, M.I. Kang, B.Y. Cha, K.W. Lee, H.Y. Son, Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid 20(8), 885–891 (2010)
F. Grob, J. Deladoey, L. Legault, L. Spigelblatt, A. Fournier, G. Vassart, G. Van Vliet, Autonomous adenomas caused by somatic mutations of the thyroid-stimulating hormone receptor in children. Horm. Res. Paediatr. 81(2), 73–79 (2014)
T.F. Davies, T. Ando, R.Y. Lin, Y. Tomer, R. Latif, Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J. Clin. Invest. 115(8), 1972–1983 (2005)
H. He, W. Li, S. Liyanarachchi, J. Jendrzejewski, M. Srinivas, R.V. Davuluri, R. Nagy, A. de la Chapelle, Genetic predisposition to papillary thyroid carcinoma: involvement of FOXE1, TSHR and a novel lincRNA gene, PTCSC2. J. Clin. Endocrinol. Metab. 100(1), 164–172 (2015)
M.S. Khan, A.A. Pandith, S.R. Masoodi, K.A. Wani, M. Ul, Hussain, S. Mudassar, Epigenetic silencing of TSHR gene in thyroid cancer patients in relation to their BRAF V600E mutation status. Endocrine 47(2), 449–455 (2014)
D.S. Cooper, G.M. Doherty, B.R. Haugen, R.T. Kloos, S.L. Lee, S.J. Mandel, E.L. Mazzaferri, B. Mclver, F. Pacini, M. Schlumberger, S.I. Sherman, D.L. Steward, R.M. Tuttle, Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19(11), 1167–1214 (2009)
G. Brabant, C. Maenhaut, J. Kohrle, G. Scheumann, H. Dralle, C. Hoang-Vu, R.D. Hesch, A. von zur Mühlen, G. Vassart, J.E. Dumont, Human thyrotropin receptor gene: expression in thyroid tumors and correlation to markers of thyroid differentiation and dedifferentiation. Mol. Cell. Endocrinol. 82(1), 7–12 (1991)
M. D’Agostino, M. Sponziello, C. Puppin, M. Celano, V. Maggisano, F. Baldan, M. Biffoni, S. Bulotta, C. Durante, S. Filetti, G. Damante, D. Russo, Different expression of TSH receptor and NIS genes in thyroid cancer: role of epigenetics. J. Mol. Endocrinol. 52(2), 121–131 (2014)
J. Gudmundsson, P. Sulem, D.F. Gudbjartsson, J.G. Jonasson, A. Sigurdsson, J.T. Bergthorsson, H. He, T. Blondal, F. Geller, M. Jakobsdottir, D.N. Magnusdottir, S. Matthiasdottir, S.N. Stacey, O.B. Skarphedinsson, H. Helgadottir, W. Li, R. Nagy, E. Aguillo, E. Faure, E. Prats, B. Saez, M. Martinez, G.I. Eyjolfsson, U.S. Bjornsdottir, H. Holm, K. Kristjansson, M.L. Frigge, H. Kristvinsson, J.R. Gulcher, T. Jansson, T. Rafnar, H. Hjartarsson, J.I. Mayordomo, A. de la Chapelle, J. Hrafnkelsson, U. Thorsteinsdottir, A. Kong, K. Stefansson, Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat. Genet. 41(4), 460–464 (2009)
J. Gudmundsson, P. Sulem, D.F. Gudbjartsson, J.G. Jonasson, G. Masson, H. He, A. Jonasdottir, A. Sigurdsson, S.N. Stacey, H. Johannsdottir, H.T. Helgadottir, W. Li, R. Nagy, M.D. Ringel, R.T. Kloos, M.C. de Visser, T.S. Plantinga, M. den Heijer, E. Aguillo, A. Panadero, E. Prats, A. Garcia-Castano, A. De Juan, F. Rivera, G.B. Walters, H. Bjarnason, L. Tryggvadottir, G.I. Eyjolfsson, U.S. Bjornsdottir, H. Holm, I. Olafsson, K. Kristjansson, H. Kristvinsson, O.T. Magnusson, G. Thorleifsson, J.R. Gulcher, A. Kong, L.A. Kiemeney, T. Jonsson, H. Hjartarson, J.I. Mayordomo, R.T. Netea-Maier, A. de la Chapelle, J. Hrafnkelsson, U. Thorsteinsdottir, T. Rafnar, K. Stefansson, Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat. Genet. 44(3), 319–322 (2012)
F. Furuya, H. Ying, L. Zhao, S.Y. Cheng, Novel functions of thyroid hormone receptor mutants: beyond nucleus-initiated transcription. Steroids 72(2), 171–179 (2007)
J. Jonklaas, H. Nsouli-Maktabi, S.J. Soldin, Endogenous thyrotropin and triiodothyronine concentrations in individuals with thyroid cancer. Thyroid 18(9), 943–952 (2008)
S. Rinaldi, M. Plummer, C. Biessy, K.K. Tsilidis, J.N. Østergaard, K. Overvad, A. Tjonneland, J. Halkjaer, M.C. Boutron-Ruault, F. Clavel-Chapelon, L. Dossus, R. Kaaks, A. Lukanova, H. Boeing, A. Trichopoulou, P. Lagiou, D. Trichopoulod, D. Palli, C. Agnoli, R. Tumino, P. Vineis, S. Panico, H.B. Bueno-de-Mesquita, P.H. Peeters, E. Weiderpass, E. Lund, J.R. Quiros, A. Agudo, E. Molina, N. Larranaga, C. Navarro, E. Ardanaz, J. Manjer, M. Almquist, M. Sandström, J. Hennings, K.T. Khaw, J. Schmidt, R.C. Travis, G. Byrnes, A. Scalbert, I. Romieu, M. Gunter, E. Riboli, S. Franceschi, Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: the EPIC study. J. Natl. Cancer. Inst. 106(6), dju097 (2014)
F.F. Maia, P.S. Matos, E.J. Pavin, D.E. Zantut-Wittmann, Thyroid imaging reporting and data system score combined with Bethesda system for malignancy risk stratification in thyroid nodules with indeterminate results on cytology. Clin. Endocrinol. 82(3), 439–444 (2015)
T.G. Strieder, M.F. Prummel, J.G. Tijssen, E. Endert, W.M. Wiersinga, Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin. Endocrinol. 59(3), 396–401 (2003)
M.R. Haymart, S.L. Glinberg, J. Liu, R.S. Sippel, J.C. Jaume, H. Chen, Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin. Endocrinol. 71(3), 434–439 (2009)
M.E. Dailey, S. Lindsay, R. Skahen, Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch. Surg. 70(2), 291–297 (1955)
T. Rago, G. Di Coscio, C. Ugolini, M. Scutari, F. Basolo, F. Latrofa, R. Romani, P. Berti, L. Grasso, L.E. Braverman, A. Pinchera, P. Vitti, Clinical features of thyroid autoimmunity are associated with thyroiditis on histology and are not predictive of malignancy in 570 patients with indeterminate nodules on cytology who had a thyroidectomy. Clin. Endocrinol. 67(3), 363–369 (2007)
F. Boi, M.L. Lai, B. Marziani, L. Minerba, G. Faa, S. Mariotti, High prevalence of suspicious cytology in thyroid nodules associated with positive thyroid autoantibodies. Eur. J. Endocrinol. 153(5), 637–642 (2005)
B. Corvilain, The natural history of thyroid autonomy and hot nodules. Ann. Endocrinol. 64(1), 17–22 (2003)
G. Treglia, P. Trimboli, F.A. Verburg, M. Luster, L. Giovanella, Prevalence of normal TSH value among patients with autonomously functioning thyroid nodule. Eur. J. Clin. Invest. 45(7), 739–744 (2015)
R. Chami, R. Moreno-Reyes, B. Corvilain, TSH measurement is not an appropriate screening test for autonomous functioning thyroid nodules: a retrospective study of 368 patients. Eur. J. Endocrinol. 170(4), 593–599 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baser, H., Topaloglu, O., Tam, A.A. et al. Higher TSH can be used as an additional risk factor in prediction of malignancy in euthyroid thyroid nodules evaluated by cytology based on Bethesda system. Endocrine 53, 520–529 (2016). https://doi.org/10.1007/s12020-016-0919-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0919-4