Abstract
Summary
Persistence to denosumab or zoledronic acid was increased compared to oral bisphosphonates.
Introduction
Denosumab and zoledronic acid are alternative therapies to oral bisphosphonates. Few studies have assessed persistence of those agents.
Methods
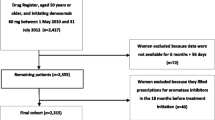
Incident users of denosumab and zoledronic acid were identified using healthcare databases of public drug insurance plan of Quebec province, Canada. Patients initiating therapy between October 1, 2008, and June 30, 2013, and aged 50 years and over were eligible. A persistence rate was assessed over a 2-year period. We assess the proportion of patients receiving the second, third, and fourth injections within a specific delay of predicted time of renewal of both agents. The predictors of non-persistence were analyzed using a Cox regression model only among women.
Results
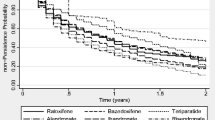
Among 12,689 incident users, 97.2 % were women. Kaplan–Meier analysis showed a slow decline of persistence after initiating zoledronic acid compared to denosumab therapy, dropping to 81.6 and 63.3 % after 1 and 2 years of follow-up using the permissive gaps of 56 days, in contrast to zoledronic acid, where persistence rate still stays at 74.8 % after 2 years of follow-up using the permissive gap of 112 days. The likelihood of non-persistence was significantly higher among new users of denosumab and zoledronic acid among older patients and year of initiation; but depression and diabetes are only predictors of non-persistence among the zoledronic group. Concomitant use of calcium and vitamin D supplements was at low level which may compromise the clinical efficacy.
Conclusion
The persistence rate to denosumab and zoledronic acid was higher to the published data of oral bisphosphonates. The second intention of treatment seems to target more severe patients which may more likely to be compliant.




Similar content being viewed by others
References
Bolland MJ, Grey AB, Gamble GD, Reid IR (2010) Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab 95(3):1174–1181
Kanis JA, Johnell O, De Laet C, et al. (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35(2):375–382
Bessette L, Jean S, Lapointe-Garant MP, et al. (2012) Direct medical costs attributable to peripheral fractures in Canadian post-menopausal women. Osteoporos Int 23(6):1757–1768
Tarride JE, Hopkins RB, Leslie WD, et al. (2012) The burden of illness of osteoporosis in Canada. Osteoporos Int 23(11):2591–2600
Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S (2009) Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int 20(9):1571–1581
Migliore A, Broccoli S, Massafra U, Cassol M, Frediani B (2013) Ranking antireabsorptive agents to prevent vertebral fractures in postmenopausal osteoporosis by mixed treatment comparison meta-analysis. Eur Rev Med Pharmacol Sci 17(5):658–667
Murad MH, Drake MT, Mullan RJ, et al. (2012) Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab 97(6):1871–1880
Morin S, Rahme E, Behlouli H, Tenenhouse A, Goltzman D, Pilote L (2007) Effectiveness of antiresorptive agents in the prevention of recurrent hip fractures. Osteoporos Int 18(12):1625–1632
Cadarette SM, Carney G, Baek D, Gunraj N, Paterson JM, Dormuth CR (2012) Osteoporosis medication prescribing in British Columbia and Ontario: impact of public drug coverage. Osteoporos Int 23(4):1475–1480
Maraka S, Kennel KA (2015) Bisphosphonates for the prevention and treatment of osteoporosis. BMJ 351:h3783
Blouin J, Dragomir A, Ste-Marie LG, Fernandes JC, Perreault S (2007) Discontinuation of antiresorptive therapies: a comparison between 1998-2001 and 2002-2004 among osteoporotic women. J Clin Endocrinol Metab 92(3):887–894
Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122(2 Suppl):S3–13
Siris ES, Pasquale MK, Wang Y, Watts NB (2011) Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res 26(1):3–11
Wade SW, Curtis JR, Yu J, et al. (2012) Medication adherence and fracture risk among patients on bisphosphonate therapy in a large United States health plan. Bone 50(4):870–875
Halpern R, Becker L, Iqbal SU, Kazis LE, Macarios D, Badamgarav E (2011) The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm 17(1):25–39
Ross S, Samuels E, Gairy K, Iqbal S, Badamgarav E, Siris E (2011) A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health 14(4):571–581
Siris ES, Harris ST, Rosen CJ, et al. (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81(8):1013–1022
Curtis JR, Yun H, Matthews R, Saag KG, Delzell E (2012) Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res (Hoboken) 64(7):1054–1060
Hadji P, Papaioannou N, Gielen E, et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int. 2015.
Hughes C (2010) ICD-9 update 2011: approaching the change to ICD-10. Fam Pract Manag 17(5):15–16
Régie de l’assurance maladie du Québec (RAMQ). Rapport annuel de gestion 2014–2015. 2015. http://www.ramq.gouv.qc.ca/SiteCollectionDocuments/citoyens/fr/rapports/rappann1415.pdf.
Moride Y, Abenhaim L (1994) Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol 47(7):731–737
Tamblyn R, Lavoie G, Petrella L, Monette J (1995) The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol 48(8):999–1009
Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M (2000) Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol 53(2):183–194
Wilchesky M, Tamblyn RM, Huang A (2004) Validation of diagnostic codes within medical services claims. J Clin Epidemiol 57(2):131–141
Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S (2013) The validity of administrative data to identify hip fractures is high—a systematic review. J Clin Epidemiol 66(3):278–285
Silverman SL, Siris E, Kendler DL, et al. (2015) Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study. Osteoporos Int 26(1):361–372
Andrade SE, Kahler KH, Frech F, Chan KA (2006) Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 15(8):565–574 discussion 575-567
Hadji P, Papaioannou N, Gielen E, et al. (2015) Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int 26(10):2479–2489
Cadarette SM, Levesque L, Mamdani M, et al. (2013) Comparison of orally administered bisphosphonate drugs in reducing the risk of hip fracture in older adults: a population-based cohort study. CMAJ Open 1(3):E97–E105
Blouin J, Dragomir A, Moride Y, Ste-Marie LG, Fernandes JC, Perreault S (2008) Impact of noncompliance with alendronate and risedronate on the incidence of nonvertebral osteoporotic fractures in elderly women. Br J Clin Pharmacol 66(1):117–127
Papaioannou A, Morin S, AM C, et al. (2010) Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 182(17):1864–1873
Burden AM, Paterson JM, Solomon DH, Mamdani M, Juurlink DN, Cadarette SM (2012) Bisphosphonate prescribing, persistence and cumulative exposure in Ontario, Canada. Osteoporos Int 23(3):1075–1082
Lee YK, Nho JH, Ha YC, Koo KH (2012) Persistence with intravenous zoledronate in elderly patients with osteoporosis. Osteoporos Int 23(9):2329–2333
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26(10):2401–2411
Foundation NO. Clinicians’s guide to prevention and treatment of osteoporosis. 2013.
Schousboe JT (2013) Adherence with medications used to treat osteoporosis: behavioral insights. Curr Osteoporos Rep 11(1):21–29
Overman RA, Gourlay ML, Deal CL. Adherence to denosumab in a large healthcare system. Arthritis and Rheumatology. Vol 66 2014:S1011.
INESSS. Portrait de l’usage des bisphosphonates et du dénosumab chez les personnes de 50 ans ou plus souffrant d’ostéoporose couvertes par le régime public d’assurance médicaments: Institut national d’excellence en santé et en services sociaux (INESSS);2014. https://www.inesss.qc.ca/fileadmin/doc/INESSS/Rapports/Medicaments/INESSS_Portrait_usage_osteoporose.pdf. Accessed 9 Sept 2016
West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A (1995) Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol 142(10):1103–1112
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The present study was approved by the research and ethics committee of INESSS.
Conflict of interest
ᅟ
Disclosures
Eric Tremblay and Marc Dorais do not have any conflicts of interest to disclose. Sylvie Perreault holds the Sanofi-Canada pharmaceutical chair on use of medications and has received research funding from Amgen in the past 2 years.
Funding sources
This work was supported by public funds from the Québec Health Ministry.
Electronic supplementary material
ESM. 1
(DOCX 42 kb)
Rights and permissions
About this article
Cite this article
Tremblay, É., Perreault, S. & Dorais, M. Persistence with denosumab and zoledronic acid among older women: a population-based cohort study. Arch Osteoporos 11, 30 (2016). https://doi.org/10.1007/s11657-016-0282-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-016-0282-3