Abstract
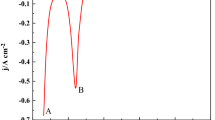
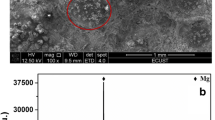
The electrode reaction of Pr(III) and coreduction of Pb(II) and Pr(III) were studied on an inert W electrode in eutectic LiCl-KCl to extract Pr from molten salt. The electrode process kinetic of Pr(III) on W electrode was firstly explored employing cyclic voltammetry (CV) and Tafel technique, such as the diffusion coefficient of Pr(III) in eutectic LiCl-KCl and the exchange current density (j0) for Pr(III)/Pr(0) couple and the electrode reaction activation energy. Then, various electrochemical techniques were used to investigate the coreduction of Pb(II) and Pr(III). Four cathode current peaks ascribed to the formation of PrxPby intermetallic compounds were detected, which indicated that the Pr could deposit on pre-deposited Pb film, react with Pb, and form PrxPby intermetallic compounds. Furthermore, the electrolytic products formed by galvanostatic electrolysis were characterized by scanning electron microscopy coupled with energy dispersive X-ray diffraction, which illustrated the formation of PrPb3 intermetallic compound in LiCl-KCl-PbCl2-PrCl3 melt.








Similar content being viewed by others
References
Hutchens RD, Wallace WE (1971) Magnetic properties of rare earth-lead intermetallics. J Solid State Chem 3:564–566. https://doi.org/10.1016/0022-4596(71)90102-2
Stalinski B (1974) Magnetic properties of some simple metallic and semimetallic compounds of electron metals with main group elements. AIP Conf Proc 18:490
Li DG, Zhou GS, Lin GF et al (2005) Anodic behavior of lead alloy with rare earth additive in sulfuric acid solution. J Chin RE Soc 23(2):224–227
Liu HT, Yang J, Liang HH et al (2001) Effect of cerium on the anodic corrosion of Pb-Ca-Sn alloy in sulfuric acid solution. J Power Sources 93(1–2):230–233. https://doi.org/10.1016/S0378-7753(00)00583-8
Chen HY, Li S, Li AJ et al (2007) Lead-samarium alloys for positive grids of valve-regulated lead-acid batteries. J Power Sources 168:79–89. https://doi.org/10.1016/j.jpowsour.2006.11.091
Li AJ, Chen YM, Chen HY et al (2009) Electrochemical behavior and application of lead-lanthanum alloys for positive grids of lead acid batteries. J Power Sources 189:1204–1211. https://doi.org/10.1016/j.jpowsour.2008.12.093
Takahisa I, Toshiyuki N, Yasuhiko I (2003) Electrochemical formation of Yb–Ni alloy films by Li codeposition method in a molten LiCl–KCl–YbCl3 system. Electrochim Acta 48(11):1531–1536. https://doi.org/10.1016/S0013-4686(03)00031-8
Bongyoung K, Donghyoung L, Junyeop L et al (2010) Electrochemical and spectroscopic investigations of Tb(III) in molten LiCl–KCl eutectic at high temperature. Electrochem Commun 12(8):1005–1008. https://doi.org/10.1016/j.elecom.2010.05.009
Córdoba GD, Laplace A, Conocar O et al (2009) Determination of the activity coefficient of Am in liquid Al by electrochemical methods. J Nucl Mater 393(3):459–464. https://doi.org/10.1016/j.jnucmat.2009.07.002
Yoon IC (1989) The integral fast reactor. Nucl Technol 88(2):129–138. https://doi.org/10.13182/NT88-129
Tadafumi K, Masatoshi I, Yuichi S et al (1997) An experimental study of molten salt electrorefining of uranium using solid iron cathode and liquid cadmium cathode for development of pyrometallurgical. J Nucl Sci Technol 34(4):384–393. https://doi.org/10.1080/18811248.1997.9733678
Hirotake M, Takuya S, Kimikazu M et al (1994) Reductive extraction behaviour of actinide and lanthanide elements in molten salt and liquid metal binary phase systems. J Alloy Compd 213-214:354–359. https://doi.org/10.1016/0925-8388(94)90930-X
Hirotake M, Hajimu Y, Sataro N et al (1997) Equilibrium distributions of actinides and lanthanides in molten chloride salt and liquid zinc binary phase system. J Nucl Mater 247:197–202. https://doi.org/10.1016/S0022-3115(98)00097-X
Gibilaro M, Massot L, Chamelot P et al (2009) Co-reduction of aluminium and lanthanide ions in molten fluorides: application to cerium and samarium extraction from nuclear wastes. Electrochim Acta 54(22):5300–5306. https://doi.org/10.1016/j.electacta.2009.01.074
Castrillejoa Y, Fernandez P, Medina J et al (2011) Electrochemical extraction of samarium from molten chlorides in pyrochemical processes. Electrochim Acta 56(24):8638–8644. https://doi.org/10.1016/j.electacta.2011.07.059
Su LL, Liu K, Liu YL et al (2014) Electrochemical behaviors of Dy(III) and its co-reduction with Al(III) in molten LiCl-KCl salts. Electrochim Acta 147:87–95. https://doi.org/10.1016/j.electacta.2014.09.095
Ping XY, Liu K, Liu YL et al (2017) Direct electrochemical preparation of Ni-Zr alloy from mixture oxides in LiCl molten salt. J Electrochem Soc 164(13):D888–D894. https://doi.org/10.1149/2.1361713jes
Castrillejo Y, Bermejo MR, Martínez AM et al (2007) Application of electrochemical techniques in pyrochemical processes- electrochemical behaviour of rare earths at W, Cd, Bi and Al electrodes. J Nucl Mater 360:32–42. https://doi.org/10.1016/j.jnucmat.2006.08.011
Gibilaro M, Massot L, Chamelot P et al (2008) Study of neodymium extraction in molten fluorides by electrochemical co-reduction with aluminium. J Nucl Mater 382:39–45. https://doi.org/10.1016/j.jnucmat.2008.09.004
Li M, Gu QQ, Han W et al (2015) Electrochemical behavior of La(III) on liquid Bi electrode in LiCl–KCl melts. Determination of thermodynamic properties of La–Bi and Li–Bi intermetallic compounds. RSC Adv 5:82471–82480. https://doi.org/10.1039/C5RA12723H
Han W, Li ZY, Li M et al (2017) Electrochemical extraction of holmium and thermodynamic properties of Ho-Bi alloys in LiCl-KCl eutectic. J Electrochem Soc 164(4):E62–E70. https://doi.org/10.1149/2.0741704jes
Tetsuya K, Tadashi I, Takashi I et al (2006) Separation behaviors of actinides from rare-earths in molten salt electrorefining using saturated liquid cadmium cathode. J Nucl Mater 357:105–114. https://doi.org/10.1016/j.jnucmat.2006.06.003
Wang L, Liu YL, Liu K et al (2015) Electrochemical extraction of cerium by forming Ce-Zn alloys in LiCl-KCl eutectic on W and liquid Zn electrodes. J Electrochem Soc 162(9):E179–E184. https://doi.org/10.1149/2.1141509jes
Han W, Li WL, Li M, Li Z, Sun Y, Yang X, Zhang M (2018) Electrochemical coreduction of Y(III) and Zn(II) and extraction of yttrium on Zn electrode in LiCl-KCl eutectic melts. J Solid State Electrchem 22(8):2435–2444. https://doi.org/10.1007/s10008-018-3956-5
Liu YL, Zhou W, Tang HB et al (2016) Diffusion coefficient of Ho3+ at liquid zinc electrode and co-reduction behaviors of Ho3+ and Zn2+ on W electrode in the LiCl-KCl eutectic. Electrochim Acta 211:313–321. https://doi.org/10.1016/j.electacta.2016.06.061
Liu YL, Ye GA, Liu K et al (2015) Electrochemical behavior of La(III) on the zinc-coated W electrode in LiCl-KCl eutectic. Electrochim Acta 168:206–215. https://doi.org/10.1016/j.electacta.2015.03.219
Luo LX, Liu YL, Liu N et al (2016) Electrochemical and thermodynamic properties of Nd (III)/Nd (0) couple at liquid Zn electrode in LiCl-KCl melt. Electrochim Acta 191:1026–1036. https://doi.org/10.1016/j.electacta.2016.01.115
Liu K, Liu YL, Chai ZF et al (2017) Evaluation of the electroextractions of Ce and Nd from LiCl-KCl molten salt using liquid Ga electrode. J Electrochem Soc 164(4):D169–D178. https://doi.org/10.1149/2.0511704jes
Castrillejo Y, Bermejo MR, Arocas DP et al (2005) Electrochemical behaviour of praseodymium (III) in molten chlorides. J Electroanal Chem 575:61–74. https://doi.org/10.1016/j.jelechem.2004.08.020
Castrillejo Y, Bermejo MR, Arocas DP et al (2005) The electrochemical behaviour of the Pr(III)/Pr redox system at Bi and Cd liquid electrodes in molten eutectic LiCl-KCl. J Electroanal Chem 579:343–358. https://doi.org/10.1016/j.jelechem.2005.03.001
Jiang T, Peng SM, Li M et al (2016) Electrochemical behavior of Pr(III) ions on a Bi-coated W electrode in LiCl-KCl melts. Acta Phys -Chim Sin 32:1708–1714
Wang YC, Li M, Han W, Zhang M, Yang Y, Sun Y, Zhao Y, Yan Y (2015) Electrochemical extraction and separation of praseodymium and erbium on reactive magnesium electrode in molten salts. J Solid State Electrchem 19:3629–3638. https://doi.org/10.1007/s10008-015-2989-2
Toshiyuki N, Hiroyuki K, Koji A et al (2005) Electrochemical formation and phase control of Pr-Ni alloys in a molten LiCl-KCl-PrCl3 system. J Electrochem Soc 152(4):C183–C189. https://doi.org/10.1149/1.1864281
Li M, Li W, Han W et al (2014) Electrochemical behavior of Pr(III) in the LiCl-KCl melt on a Ni electrode. Chem J Chinese U 35(12):2662–2667. https://doi.org/10.7503/cjcu20140405
Li M, Wang J, Han W et al (2017) Electrochemical formation and thermodynamic evaluation of Pr-Zn intermetallic compounds in LiCl-KCl eutectic melts. Electrochim Acta 228:299–307. https://doi.org/10.1016/j.electacta.2017.01.070
Berzins T, Delahay P (1953) Theory of electrolysis at constant current in unstirred solution. II Consecutive electrochemical reactions. J Am Chem Soc 75(17):4205–4213. https://doi.org/10.1021/ja01113a022
Castrillejo Y, Bermejo MR, Diaz P et al (2005) Electrochemical behaviour of praseodymium (III) in molten chlorides. J Electroanal Chem 575:61–74. https://doi.org/10.1016/j.jelechem.2004.08.020
Bard AJ, Faulkner LR (2001) Electrochemical methods, fundamentals and applications, 2nd edn. Wiley, New York
Lim KH, Yun JI (2019) Study on the exchange current density of lanthanide chlorides in LiCl-KCl molten salt. Electrochim Acta 295:577–583. https://doi.org/10.1016/j.electacta.2018.10.104
Kim KR, Choi SY, Kim JG et al (2015) Electrochemical investigation of exchange current density of uranium and rare-earths couples (M3+/M0) in LiCl-KCl eutectic electrolyte. Int J Electrochem Sci 10:7660–7670
Wang YC, Liu YH, Li M, Han W, Liu Y, Liu Y (2019) Extraction of gadolinium on Cu electrode from LiCl-KCl melts by formation of Cu-Gd alloys. Ionics 25:1897–1909. https://doi.org/10.1007/s11581-018-2762-5
Han W, Wang WJ, Dong YC et al (2018) The kinetics process of a Pb(II)/Pb(0) couple and selective fabrication of Li-Pb alloys in LiCl-KCl melts. RSC Adv 8(53):30530–30538. https://doi.org/10.1039/C8RA06329J
Zydzik M, Smids M, Vanende MA et al (2014) Critical thermodynamic evaluation and optimization of the Pb-Pr, Pb-Nd, Pb-Tb and Pb-Dy systems. CALPHAD: Computer Coupling of Phase Diagrams and Thermochemistry 46:1–17
Funding
The work was financially supported by the National Natural Science Foundation of China (21876034, 11875116, 21790373, 11675044, and 11575047).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, M., Sun, Z., Guo, D. et al. Electrode reaction of Pr(III) and coreduction of Pr(III) and Pb(II) on W electrode in eutectic LiCl-KCl. Ionics 26, 3901–3909 (2020). https://doi.org/10.1007/s11581-020-03518-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03518-4