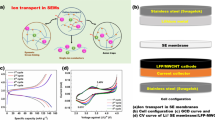
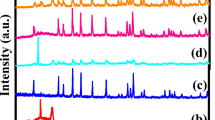
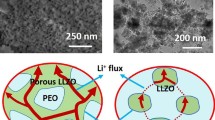
Abstract
Three types of inorganic electrolytes [Li10GeP2S12 (LGPS), 75Li2S·24P2S5·1P2O5 (LPOS), Li1.5Al0.5Ge1.5(PO4)3 (LAGP)] with different particle sizes and electrochemical properties are selected as active fillers incorporated into poly(ethylene oxide) (PEO) matrix to fabricate hybrid solid electrolytes. The optimum composition of each filler is found in consideration of ionic conductivity. Their electrochemical characteristics are investigated. The optimal conductivities are 1.60 × 10−5, 1.18 × 10−5, and 2.12 × 10−5 S cm−1 at room temperature for PEO-1%LGPS, PEO-1%LPOS, and PEO-20%LAGP, respectively. The electrochemical stability windows of these hybrid solid electrolytes are all above 5 V (vs. Li+/Li). The results show that these fillers have positive effects on the ionic conductivity, lithium ion transference number, and electrochemical stability. The relationship between the type of filler and electrochemical properties has been investigated. All-solid-state cells LiFePO4/Li are fabricated and present fascinating electrochemical performance with high capacity retention and good cycling stability. This work provides promising electrolytes prepared by a simple method.











Similar content being viewed by others
References
Goodenough JB, Kim Y (2011) Challenges for rechargeable batteries. J Power Sources 196:6688–6694
Bouchet R, Maria S, Meziane R, Aboulaich A, Lienafa L, Bonnet JP, Phan TNT, Bertin D, Gigmes D, Devaux D, Denoyel R, Armand M (2013) Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat Mater 12:452–457
Nguyen CA, Argun AA, Hammond PT, Lu XH, Lee PS (2011) Layer-by-layer assembled solid polymer electrolyte for electrochromic devices. Chem Mat 23:2142–2149
Christie AM, Lilley SJ, Staunton E, Andreev YG, Bruce PG (2005) Increasing the conductivity of crystalline polymer electrolytes. Nature 433:50–53
Thokchom JS, Gupta N, Kumar B (2008) Superionic conductivity in a lithium aluminum germanium phosphate glass-ceramic. J Electrochem Soc 155:A915–A920
Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A (2011) A lithium superionic conductor. Nat Mater 10:682–686
Minami K, Hayashi A, Ujiie S, Tatsumisago M (2011) Electrical and electrochemical properties of glass-ceramic electrolytes in the systems Li2S-P2S5-P2S3 and Li2S-P2S5-P2O5. Solid State Ionics 192:122–125
Scrosati B (1993) Applications of electroactive polymers. Chapman & Hall, London
Gray FM (1997) Polymer electrolytes, in: RSC materials monographs. The Royal Society of Chemistry, Cambridge
Kim JG, Son B, Mukherjee S, Schuppert N, Bates A, Kwon O, Choi MJ, Chung HY, Park S (2015) A review of lithium and non-lithium based solid state batteries. J Power Sources 282:299–322
Inda Y, Katoh T, Baba M (2007) Development of all-solid lithium-ion battery using Li-ion conducting glass-ceramics. J Power Sources 174:741–744
Nan CW, Fan LZ, Lin YH, Cai Q (2003) Enhanced ionic conductivity of polymer electrolytes containing nanocomposite SiO2 particles. Phys Rev Lett 91:4
Choi JH, Lee CH, Yu JH, Doh CH, Lee SM (2015) Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix. J Power Sources 274:458–463
Jung YC, Lee SM, Choi JH, Jang SS, Kim DW (2015) All solid-state lithium batteries assembled with hybrid solid electrolytes. J Electrochem Soc 162:A704–A710
Yin JY, Yao XY, Peng G, Yang J, Huang Z, Liu D, Tao YC, Xu XX (2015) Influence of the Li-Ge-P-S based solid electrolytes on NCA electrochemical performances in all-solid-state lithium batteries. Solid State Ionics 274:8–11
Tao YC, Chen SJ, Liu D, Peng G, Yao XY, Xu XX (2016) Lithium superionic conducting oxysulfide solid electrolyte with excellent stability against lithium metal for all-solid-state cells. J Electrochem Soc 163:A96–A101
Yang J, Huang Z, Huang BX, Zhou J, Xu XX (2015) Influence of phosphorus sources on lithium ion conducting performance in the system of Li2O-Al2O3-GeO2-P2O5 glass-ceramics. Solid State Ionics 270:61–65
Evans J, Vincent CA, Bruce PG (1987) Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28:2324–2328
Masoud EM, El-Bellihi AA, Bayoumy WA, Mousa MA (2013) Organic-inorganic composite polymer electrolyte based on PEO-LiClO4 and nano-Al2O3 filler for lithium polymer batteries: dielectric and transport properties. J Alloy Compd 575:223–228
Kim JW, Ji KS, Lee JP, Park JW (2003) Electrochemical characteristics of two types of PEO-based composite electrolyte with functional SiO2. J Power Sources 119:415–421
Chinnasamy RM, Chihiro Y, Fabio R, Bernhard R (2011) Correlation between micro-structural properties and ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 ceramics. J Power Sources 196:6456–6464
Wetjen M, Navarra MA, Panero S, Passerini S, Scrosati B, Hassoun J (2013) Composite poly(ethylene oxide) electrolytes plasticized by N-alkyl-N-butylpyrrolidinium bis(trifluoromethanesulfonyl)imide for lithium batteries. ChemSusChem 6:1037–1043
Plylahan N, Letiche M, Barr MKS, Djenizian T (2014) All-solid-state lithium-ion batteries based on self-supported titania nanotubes. Electrochem Commun 43:121–124
Srivastava S, Schaefer JL, Yang Z, Tu Z, Archer LA (2014) 25th anniversary article: polymer-particle composites: phase stability and applications in electrochemical energy storage. Adv Mater 26:201–234
Abraham KM, Alamgir M, Moulton RD (1991) Polyphosphazene-poly(olefin oxide) mixed polymer electrolytes: II. Characterization of MEEP/PPO(LiX)n. J Electrochem Soc 138:921–927
Park CH, Kim DW, Prakash J, Sun YK (2003) Electrochemical stability and conductivity enhancement of composite polymer electrolytes. Solid State Ionics 159:111–119
Marcinek M, Bac A, Lipka P, Zalewska A, Zukowska G, Borkowska R, Wieczorek W (2000) Effect of filler surface group on ionic interactions in PEG-LiClO4-Al2O3 composite polyether electrolytes. J Phys Chem B 104:11088–11093
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458
Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (Grant No. 51502317), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant Nos. XDA09010201 and XDA09010203), the Key Scientific and Technological Innovation Team Project of Zhejiang province (Grant No. 2013PT16), and Natural Science Foundation of Ningbo (Grant Nos. 2015A610238 and 2015A610027).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Zhao, Y., Yang, J. et al. Hybrid solid electrolytes with excellent electrochemical properties and their applications in all-solid-state cells. Ionics 23, 2603–2611 (2017). https://doi.org/10.1007/s11581-016-1905-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1905-9