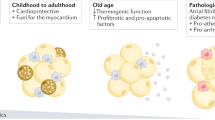
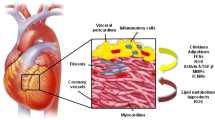
Abstract
Worldwide, cardiovascular diseases (CVDs) are a leading cause of mortality. While in many westernized societies there has been a decrease prevalence of smoking and that a special emphasis has been put on the urgency to control the, so called, classical risk factors, it is more and more recognized that there remains a residual risk, which contributes to the development of CVDs. Imaging studies conducted over two decades have highlighted that the accumulation of ectopic visceral fat is associated with a plethora of metabolic dysfunctions, which have complex and intertwined interactions and participate to the development/progression/events of many cardiovascular disorders. The contribution of visceral ectopic fat to the development of coronary artery disease (CAD) is now well established, while in the last several years emerging evidence has pointed out that accumulation of harmful ectopic fat is associated with other cardiovascular disorders such as calcific aortic valve disease (CAVD), atrial fibrillation and left ventricular dysfunction. We review herein the key molecular processes linking the accumulation of ectopic fat to the development of CVDs. We have attempted, whenever possible, to use a translational approach whereby the pathobiology processes are linked to clinical observations.


Similar content being viewed by others
References
Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2011 update: a report from the American heart association. Circulation. 2011;123(4):e18–e209.
Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28(6):1039–49.
Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7.
Mathieu P, Pibarot P, Larose E, et al. Visceral obesity and the heart. Int J Biochem Cell Biol. 2008;40(5):821–36.
Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97(7):2482–8.
Wilson PW, D’Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–72.
Lemieux I, Pascot A, Prud’homme D, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21(6):961–7.
Arsenault BJ, Lemieux I, Despres JP, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ. 2010;182(13):1427–32.
Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92(2):399–404.
Mauriege P, Despres JP, Moorjani S, et al. Abdominal and femoral adipose tissue lipolysis and cardiovascular disease risk factors in men. Eur J Clin Invest. 1993;23(11):729–40.
Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–50.
Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87(4):407–16.
Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32.
Parhami F, Tintut Y, Ballard A, et al. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88(9):954–60.
Zeadin M, Butcher M, Werstuck G, et al. Effect of leptin on vascular calcification in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29(12):2069–75.
Slawik M, Vidal-Puig AJ. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev. 2006;5(2):144–64.
Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42–72.
Lee Y, Wang MY, Kakuma T, et al. Liporegulation in diet-induced obesity. the antisteatotic role of hyperleptinemia. J Biol Chem. 2001;276(8):5629–35.
Kim JK, Gavrilova O, Chen Y, et al. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275(12):8456–60.
Mathieu P, Boulanger MC. Basic Mechanisms of calcific aortic valve disease. Can J Cardiol. 2014;30(9):982–93.
Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–38.
Despres JP. Inflammation and cardiovascular disease: is abdominal obesity the missing link? Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S22–4.
Blackburn P, Despres JP, Lamarche B, et al. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring). 2006;14(10):1747–54.
Degerman E, Landstrom TR, Wijkander J, et al. Phosphorylation and activation of hormone-sensitive adipocyte phosphodiesterase type 3B. Methods. 1998;14(1):43–53.
Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18(4):401–8.
Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12.
Arsenault BJ, Pibarot P, Despres JP. The quest for the optimal assessment of global cardiovascular risk: are traditional risk factors and metabolic syndrome partners in crime? Cardiology. 2009;113(1):35–49.
Arsenault BJ, Cartier A, Cote M, et al. Body composition, cardiorespiratory fitness, and low-grade inflammation in middle-aged men and women. Am J Cardiol. 2009;104(2):240–6.
Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30(3):261–77.
Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107(3):398–404.
Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103(9):1194–7.
Torzewski J, Torzewski M, Bowyer DE, et al. C-reactive protein frequently colocalizes with the terminal complement complex in the intima of early atherosclerotic lesions of human coronary arteries. Arterioscler Thromb Vasc Biol. 1998;18(9):1386–92.
Okamoto Y, Arita Y, Nishida M, et al. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32(2):47–50.
Ouchi N, Kihara S, Funahashi T, et al. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14(6):561–6.
Cote M, Mauriege P, Bergeron J, et al. Adiponectinemia in visceral obesity: impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J Clin Endocrinol Metab. 2005;90(3):1434–9.
Maahs DM, Ogden LG, Kinney GL, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111(6):747–53.
Mohty D, Pibarot P, Cote N, et al. Hypoadiponectinemia is associated with valvular inflammation and faster disease progression in patients with aortic stenosis. Cardiology. 2011;118(2):140–6.
Denzel MS, Scimia MC, Zumstein PM, et al. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120(12):4342–52.
Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12.
Reilly MP, Lehrke M, Wolfe ML, et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111(7):932–9.
Mohty D, Pibarot P, Despres JP, et al. Age-related differences in the pathogenesis of calcific aortic stenosis: the potential role of resistin. Int J Cardiol. 2010;142(2):126–32.
Garrison RJ, Kannel WB, Stokes III J, et al. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16(2):235–51.
Mathieu P, Poirier P, Pibarot P, et al. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53(4):577–84.
McAllister-Lucas LM, Ruland J, Siu K, et al. CARMA3/Bcl10/MALT1-dependent NF-kappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci U S A. 2007;104(1):139–44.
Choudhary S, Lu M, Cui R, et al. Involvement of a novel Rac/RhoA guanosine triphosphatase-nuclear factor-kappaB inducing kinase signaling pathway mediating angiotensin II-induced RelA transactivation. Mol Endocrinol. 2007;21(9):2203–17.
Mazzolai L, Duchosal MA, Korber M, et al. Endogenous angiotensin II induces atherosclerotic plaque vulnerability and elicits a Th1 response in ApoE−/− mice. Hypertension. 2004;44(3):277–82.
Fujisaka T, Hoshiga M, Hotchi J, et al. Angiotensin II promotes aortic valve thickening independent of elevated blood pressure in apolipoprotein-E deficient mice. Atherosclerosis. 2013;226(1):82–7.
Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–88.
Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–61.
Derbali H, Bosse Y, Cote N, et al. Increased biglycan in aortic valve stenosis leads to the overexpression of phospholipid transfer protein via Toll-like receptor 2. Am J Pathol. 2010;176(6):2638–45.
Zeng Q, Jin C, Ao L, et al. Cross-talk between the Toll-like receptor 4 and Notch1 pathways augments the inflammatory response in the interstitial cells of stenotic human aortic valves. Circulation. 2012;126(11 Suppl 1):S222–30.
Cani PD, Osto M, Geurts L, et al. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3(4):279–88.
Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86(2):211–8.
Finco TS, Beg AA, Baldwin Jr AS. Inducible phosphorylation of I kappa B alpha is not sufficient for its dissociation from NF-kappa B and is inhibited by protease inhibitors. Proc Natl Acad Sci U S A. 1994;91(25):11884–8.
Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293(5535):1673–7.
Sui Y, Park SH, Xu J, et al. IKKbeta links vascular inflammation to obesity and atherosclerosis. J Exp Med. 2014;211(5):869–86.
El HD, Boulanger MC. Mahmut A, et al. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: implication for calcific aortic valve disease. J Mol Cell Cardiol. 2014;72:146–56.
Babu AN, Meng X, Zou N, et al. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg. 2008;86(1):71–6.
Eder K, Baffy N, Falus A, et al. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58(11):727–36.
Melendez GC, McLarty JL, Levick SP, et al. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56(2):225–31.
Pascot A, Lemieux I, Prud’homme D, et al. Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity. J Lipid Res. 2001;42(12):2007–14.
Lemieux I, Couillard C, Pascot A, et al. The small, dense LDL phenotype as a correlate of postprandial lipemia in men. Atherosclerosis. 2000;153(2):423–32.
Lamarche B, Tchernof A, Mauriege P, et al. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279(24):1955–61.
Mohty D, Pibarot P, Despres JP, et al. Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol. 2008;28(1):187–93.
Cote C, Pibarot P, Despres JP, et al. Association between circulating oxidised low-density lipoprotein and fibrocalcific remodeling of the aortic valve in aortic stenosis. Heart. 2008;94(9):1175–80.
Mahmut A, Boulanger MC, El HD, et al. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease: implications for valve mineralization. J Am Coll Cardiol. 2014;63(5):460–9.
Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343–56.
Goldbourt U, Yaari S, Medalie JH. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler Thromb Vasc Biol. 1997;17(1):107–13.
Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96(12):1221–32.
Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–72.
Parhami F, Basseri B, Hwang J, et al. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002;91(7):570–6.
Mahmut A, Boulanger MC, Fournier D, et al. Lipoprotein lipase in aortic valve stenosis is associated with lipid retention and remodeling. Eur J Clin Invest. 2013;43(6):570–8.
Huang Y, DiDonato JA, Levison BS, et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med. 2014;20(2):193–203.
Bots ML, Visseren FL, Evans GW, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370(9582):153–60.
Rader DJ. deGoma EM. Future of cholesteryl ester transfer protein inhibitors. Annu Rev Med. 2014;65:385–403.
St-Pierre AC, Cantin B, Dagenais GR, et al. The triglyceride/high-density lipoprotein cholesterol ratio, the small dense low-density lipoprotein phenotype, and ischemic heart disease risk. Metab Syndr Relat Disord. 2004;2(1):57–64.
Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299(13):1547–60.
Saremi A, Schwenke DC, Buchanan TA, et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2013;33(2):393–9.
Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298(10):1180–8.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71.
Endo Y, Suzuki M, Yamada H, et al. Thiazolidinediones enhance sodium-coupled bicarbonate absorption from renal proximal tubules via PPARgamma-dependent nongenomic signaling. Cell Metab. 2011;13(5):550–61.
Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54.
Gogebakan O, Kohl A, Osterhoff MA, et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the diet, obesity, and genes (DiOGenes) study: a randomized, controlled trial. Circulation. 2011;124(25):2829–38.
Glantz S, Gonzalez M. Effective tobacco control is key to rapid progress in reduction of non-communicable diseases. Lancet. 2012;379(9822):1269–71.
Acknowledgments
The work of the authors is supported by the Heart and Stroke Foundation of Canada, Fonds de Recherche Nature et Technologie Québec and CIHR grants MOP245048, MOP114893 and the Quebec Heart and Lung Institute Fund. P. Mathieu is a research scholar from the Fonds de Recherche en Santé du Québec, Montreal, Québec, Canada.
Conflict of interest
J.P. Després has served as a speaker for Abbott Laboratories, AstraZeneca, Solvay Pharma, GlaxoSmithKline, and Pfizer Canada Inc.; has received research funding from Eli Lilly Canada; and has served on the advisory boards of Novartis, Theratechnologies, Torrent Pharmaceuticals Ltd., and Sanofi-Aventis. P. Mathieu has patent applications for the treatment of calcific aortic valve disease. M.C. Boulanger reports no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathieu, P., Boulanger, MC. & Després, JP. Ectopic visceral fat: A clinical and molecular perspective on the cardiometabolic risk. Rev Endocr Metab Disord 15, 289–298 (2014). https://doi.org/10.1007/s11154-014-9299-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-014-9299-3