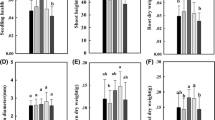
Abstract
Melatonin has different functions in plant growth and development, especially in the protection of plants suffering from various forms of abiotic stress. We explored the effect of melatonin priming on photosynthetic activity of tomato (Lycopersicon esculentum L.) leaves. Our results showed that 100 µM is the optimal concentration used for alleviation of the damage to photosynthetic apparatus. Melatonin priming both in the form of leaf spray and direct root application was found to reduce the damage to photosynthetic apparatus, and increase the electron transfer rate and quantum yield of PSI and PSII photochemistry, to protect the thylakoid membrane from damage caused by low-temperature stress. Our study provides fundamental information for further research on the molecular mechanism of melatonin function in regulating photosynthesis.
Similar content being viewed by others
Abbreviations
- C a :
-
ambient CO2 concentration
- C i :
-
intercellular CO2 concentration
- Chl:
-
chlorophyll
- CK:
-
control
- CK0:
-
plants were grown at normal temperature (day and night temperature of 25/15°C) for 3 d after pretreatment by spraying water on leaves and applying 50 mL of water on roots
- CK1:
-
plants were grown at low temperature (day and night temperature of 15/6°C) for 3 d after pretreatment by spraying water on leaves and applying 50 mL of water on roots
- E :
-
transpiration rate
- ETRI :
-
electron transfer rate of PSI
- ETRII :
-
electron transfer rate of PSII
- Fv/Fm :
-
the maximum photochemical quantum yield of PSII
- Fv′/Fm′:
-
the efficiency of excitation energy capture by open PSII reaction center
- g s :
-
stomatal conductance
- L100:
-
plants were grown at low temperature (day and night temperature of 15/6°C) for 3 d after pretreatment by leaf spraying 100 µM melatonin on leaves and applying 50 mL of water on roots
- Ls :
-
stomatal limitation
- MDA:
-
malondialdehyde
- NPQ:
-
nonphotochemical quenching
- Pm :
-
PSI content
- P N :
-
net photosynthetic rate
- PSI-CEF:
-
cyclic electron flow around PSI
- qP :
-
photochemical quenching coefficient
- R100:
-
plants were grown at low temperature (day and night temperature of 15/6°C) for 3 d after pretreatment by spraying water on leaves and applying 100 mL of melatonin on roots
- RLC:
-
rapid light curve
- RNS:
-
reactive nitrogen species
- ROS:
-
reactive oxygen species
- WUE:
-
water-use efficiency (= PN/E)
- YI :
-
quantum yield of PSI photochemistry
- YII :
-
actual quantum yield of PSII photochemistry for light-adapted state
- YNA :
-
quantum yield of nonphotochemical energy dissipation due to acceptor side limitation
- YND :
-
quantum yield of nonphotochemical energy dissipation due to donor side limitation
- YNPQ :
-
quantum yield of regulatory energy dissipation
- YNO :
-
quantum yield of nonregulatory energy dissipation.
References
Ahn T.K., Avenson T.J., Ballottari M. et al.: Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein.–Science 320: 794–797, 2008.
Arnao M.B., Hernández-Ruiz J.: Functions of melatonin in plants: a review.–J. Pineal Res. 59: 133–150, 2015.
Arnao M.B., Hernández-Ruiz J.: Melatonin: plant growth regulator and/or biostimulator during stress?–Trends Plant Sci. 19: 789–797, 2014.
Back K., Tan D.X., Reiter R.J.: Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts.–J. Pineal Res. 61:426–437, 2016.
Baránková B., Lazár D., Nauš J.: Analysis of the effect of chloroplast arrangement on optical properties of green tobacco leaves.–Remote Sens. Environ. 174: 181–196, 2016.
Beilby M.J., Turi C.E., Baker T.C. et al.: Circadian changes in endogenous concentrations of indole-3-acetic acid, melatonin, serotonin, abscisic acid and jasmonic acid in Characeae (Chara australis Brown).–Plant Signal Behav. 10: e1082697, 2015.
Borges A.A., Jiménez-Arias D., Expósito-Rodríguez M. et al.: Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms.–Front. Plant Sci. 5: 642, 2014.
Derks A., Schaven K., Bruce D.: Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change.–BBA-Bioenergetics 1847: 468–485, 2015.
Fan D.Y., Ye Z.P., Wang S.C. et al.: Multiple roles of oxygen in the photoinactivation and dynamic repair of Photosystem II in spinach leaves.–Photosynth. Res. 127: 307–319, 2016.
Fan J., Hu Z., Xie Y. et al.: Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass.–Front. Plant Sci. 6: 925, 2015.
Gururani M.A., Venkatesh J., Tran L.S.P.: Regulation of photosynthesis during abiotic stress-induced photoinhibition.–Mol Plant. 8: 1304–1320, 2015.
Hao J., Gu F., Zhu J. et al.: Low night temperature affects the phloem ultrastructure of lateral branches and raffinose family oligosaccharide (RFO) accumulation in RFO-transporting plant melon (Cucumismelo L.) during fruit expansion.–Plos One 11: e0160909, 2016.
Hu Z.R., Fan J.B., Xie Y. et al.: Comparative photosynthetic and metabolic analyses reveal mechanism of improved cold stress tolerance in bermudagrass by exogenous melatonin.–Plant Physiol. Bioch. 100: 94–104, 2016.
Johnson G.N., Lawson T., Murchie E.H. et al.: Photosynthesis in variable environments.–J. Exp. Bot. 66: 2371–2372, 2015.
Lazár D., Murch S.J., Beilby M.J. et al.: Exogenous melatonin affects photosynthesis in characeae Chara australis.–Plant Signal. Behav. 8: e23279, 2013.
Lazár D.: Parameters of photosynthetic energy partitioning.–J. Plant Physiol. 175: 131–147, 2015.
Lei Y., Zheng Y., Dai K. et al.: Different responses of photosystem I and photosystem II in three tropical oilseed crops exposed to chilling stress and subsequent recovery.–Trees 28: 923–933, 2014.
Li X., Tan D.X., Jiang D. et al.: Melatonin enhances cold tolerance in drought-primed wild-type and abscisic aciddeficient mutant barley.–J. Pineal Res. 61: 328–339, 2016.
Liu J., Wang W., Wang L. et al.: Exogenous melatonin improves seedling health index and drought tolerance in tomato.–Plant Growth Regul. 77: 317–326, 2015a.
Liu N., Jin Z.Y., Wang S.S. et al.: Sodic alkaline stress mitigation with exogenous melatonin involves reactive oxygen metabolism and ion homeostasis in tomato.–Sci. Hortic.-Amsterdam 181: 18–25, 2015b.
Liu Y.F., Zhang G.X., Qi M.F. et al.: Effects of calcium on photosynthesis, antioxidant system, and chloroplast ultrastructure in tomato leaves under low night temperature stress.–J. Plant Growth Regul. 34: 263–273, 2015c.
Lu T., Meng Z., Zhang G. et al.: Sub-high temperature and high light intensity induced irreversible inhibition on photosynthesis system of tomato plant (Solanum lycopersicum L.).–Front. Plant Sci. 8: 365, 2017.
Lyu H., Lazár D.: Modeling the light-induced electric potential difference (ΔΨ), the pH difference (ΔpH) and the proton motive force across the thylakoid membrane in C3 leaves.–J. Theor Biol. 413: 11–23, 2017.
Nawaz M.A., Huang Y., Bie Z. et al.: Melatonin: current status and future perspectives in plant science.–Front. Plant Sci. 6: 1230, 2015.
Nishiyama Y., Allakhverdiev S.I., Murata N.: Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II.–Physiol. Plantarum 142: 35–46, 2011.
Reiter R.J., Tan D.X., Zhou Z.: Phytomelatonin: assisting plants to survive and thrive.–Molecules 20: 7396–7437, 2015.
Roach T., Krieger-Liszkay A.: Regulation of photosynthetic electron transport and photoinhibition.–Curr Protein Pept. Sc. 15: 351–362, 2014.
Savvides A., Ali S., Tester M., Fotopoulos V.: Chemical priming of plants against multiple abiotic stresses: Mission Possible?–Trends Plant Sci. 21: 329–340, 2016.
Schreiber U., Klughammer C.: New accessory for the Dual-PAM-100: The P515/535 module and examples of its application.–PAM Appl. Notes 1: 1–10, 2008a.
Schreiber U., Klughammer C.: Saturation pulse method for assessment of energy conversion in PSI.–PAM Appl. Notes 1: 11–14, 2008b.
Sejima T., Takagi D., Fukayama H. et al.: Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves.–Plant Cell Physiol. 55: 1184–1193, 2014.
Suorsa M.: Cyclic electron flow provides acclimatory plasticity for the photosynthetic machinery under various environmental conditions and developmental stages.–Front. Plant Sci. 6: 800, 2015.
Suzuki K., Ohmori Y., Ratel E.: High root temperature blocks both linear and cyclic electron transport in the dark during chilling of the leaves of rice seedlings.–Plant Cell Physiol. 52: 1697–1707, 2011.
Takahashi S., Milward S.E., Fan D.Y. et al.: How does cyclic electron flow alleviate photoinhibition in Arabidopsis?–Plant Physiol. 149: 1560–1567, 2009.
Wang P., Sun X., Li C. et al.: Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple.–J. Pineal Res. 54: 292–302, 2013.
Yang S.L., Lan S.S., Deng F.F. et al.: Effects of calcium and calmodulin antagonists on chilling stress-induced proline accumulation in Jatropha curcas L.–J. Plant Growth Regul. 35: 815–826, 2016.
Zhang G., Liu Y., Ni Y. et al.: Exogenous calcium alleviates low night temperature stress on the photosynthetic apparatus of tomato leaves.–PLoS One 9: e97322, 2014a.
Zhang J., Jiang X.D., Li T.L. et al.: Photosynthesis and ultrastructure of photosynthetic apparatus in tomato leaves under elevated temperature.–Photosynthetica 52: 430–436, 2014b.
Zhang X., da Silva J.A.T., Niu M. et al.: Physiological and transcriptomic analyses reveal a response mechanism to cold stress in Santalum album L. leaves.–Sci Rep. 7: 42165, 2017.
Zhao H., Ye L., Wang Y. et al.: Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle.–Front. Plant Sci. 7: 1814, 2016.
Zhou X., Zhao H., Cao K. et al.: Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress.–Front. Plant Sci. 7: 1823, 2016.
Zhu J.K.: Abiotic stress signaling and responses in plants.–Cell 167: 313–324, 2016.
Ziogas V., Tanou G., Belghazi M. et al.: Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants.–Plant Mol. Biol. 89: 433–450, 2015.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: This work was supported by the National Key Research Program of China (2016YFD0201004); National Natural Science Foundation of China (Grant No. 31301813) and China Agriculture Research System (Grant No. CARS-25).
Rights and permissions
About this article
Cite this article
Yang, X.L., Xu, H., Li, D. et al. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 56, 884–892 (2018). https://doi.org/10.1007/s11099-017-0748-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-017-0748-6