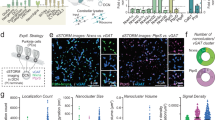
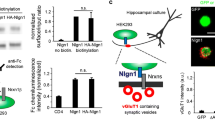
Formation and maintenance of a neuronal network is based on a balance between plasticity and stability of synaptic connections. Several molecules have been found to regulate the maintenance of excitatory synapses but nothing is known about the molecular mechanisms involved in synaptic stabilization versus disassembly at inhibitory synapses. Here, we demonstrate that Nogo-A, which is well known to be present in myelin and inhibit growth in the adult CNS, is present in inhibitory presynaptic terminals in cerebellar Purkinje cells at the time of Purkinje cell-Deep Cerebellar Nuclei (DCN) inhibitory synapse formation and is then downregulated during synapse maturation. We addressed the role of neuronal Nogo-A in synapse maturation by generating several mouse lines overexpressing Nogo-A, starting at postnatal ages and throughout adult life, specifically in cerebellar Purkinje cells and their terminals. The overexpression of Nogo-A induced a progressive disassembly, retraction and loss of the inhibitory Purkinje cell terminals. This led to deficits in motor learning and coordination in the transgenic mice. Prior to synapse disassembly, the overexpression of neuronal Nogo-A led to the downregulation of the synaptic scaffold proteins spectrin, spectrin-E and β-catenin in the postsynaptic neurons. Our data suggest that neuronal Nogo-A might play a role in the maintenance of inhibitory synapses by modulating the expression of synaptic anchoring molecules.








Similar content being viewed by others
References
Abe, K., Chisaka, O., Van Roy, F., and Takeichi, M. (2004). Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat. Neurosci. 7, 357–363
Bockers, T. M., Mameza, M. G., Kreutz, M. R., Bockmann, J., Weise, C., Buck, F., Richter, D., Gundelfinger, E. D., and Kreienkamp, H. J. (2001). Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin. J. Biol. Chem. 276, 40104–40112
Braga, V. M. (2002). Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 14, 546–556
Buffo, A., Holtmaat, A. J., Savio, T., Verbeek, J. S., Oberdick, J., Oestreicher, A. B., Gispen, W. H., Verhaagen, J., Rossi, F., and Strata, P. (1997). Targeted overexpression of the neurite growth-associated protein B-50/GAP-43 in cerebellar Purkinje cells induces sprouting after axotomy but not axon regeneration into growth-permissive transplants. J. Neurosci. 17, 8778–8791
Caroni, P. and Schwab, M. E. (1988). Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 1, 85–96
Chen, M. S., Huber, A. B., Van Der Haar, M. E., Frank, M., Schnell, L., Spillmann, A. A., Christ, F., and Schwab, M. E. (2000). Nogo-A is a myelin-associated neurite outgrowth inhibitor and an anTrends in Genetics en for monoclonal antibody IN-1. Nature 403, 434–439
Chih, B., Engelman, H., and Scheiffele, P. (2005). Control of excitatory and inhibitory synapse formation by neuroligins. Science 307, 1324–1328
Contractor, A., Rogers, C., Maron, C., Henkemeyer, M., Swanson, G. T., and Heinemann, S. F. (2002). Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science 296, 1864–1869
De Zeeuw, C. I., Hansel, C., Bian, F., Koekkoek, S. K., Van Alphen, A. M., Linden, D. J., and Oberdick, J. (1998). Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron 20, 495–508
Dimou, L., Schnell, L., Montani, L., Duncan, C., Simonen, M., Schneider, R., Liebscher, T., Gullo, M., and Schwab, M. E. (2006). Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J. Neurosci. 26, 5591–5603
Dirks, P., Thomas, U., and Montag, D. (2006). The cytoplasmic domain of NrCAM binds to PDZ domains of synapse-associated proteins SAP90/PSD95 and SAP97. Eur. J. Neurosci. 24, 25–31
Dobie, K., Mehtali, M., Mcclenaghan, M., and Lathe, R. (1997). Variegated gene expression in mice. Trends Genet. 13, 127–130
Dodd, D. A., Niederoest, B., Bloechlinger, S., Dupuis, L., Loeffler, J. P., and Schwab, M. E. (2005). Nogo-A, -B, and -C are found on the cell surface and interact together in many different cell types. J. Biol. Chem. 280, 12494–12502
Eaton, B. A. and Davis, G. W. (2005). LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron 47, 695–708
Eaton, B. A., Fetter, R. D., and Davis, G. W. (2002). Dynactin is necessary for synapse stabilization. Neuron 34, 729–741
Fritschy, J. M., Schweizer, C., Brunig, I., and Luscher, B. (2003). Pre- and post-synaptic mechanisms regulating the clustering of type A gamma-aminobutyric acid receptors (GABAA receptors). Biochem. Soc. Trans. 31, 889–892
Fritschy, J. M., Weinmann, O., Wenzel, A., and Benke, D. (1998). Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by anTrends in Genetics en-retrieval immunohistochemistry. J. Comp. Neurol. 390, 194–210
Garin, N. and Escher, G. (2001). The development of inhibitory synaptic specializations in the mouse deep cerebellar nuclei. Neuroscience 105, 431–441
Garin, N., Hornung, J. P., and Escher, G. (2002). Distribution of postsynaptic GABA(A) receptor aggregates in the deep cerebellar nuclei of normal and mutant mice. J. Comp. Neurol. 447, 210–217
Gao, P. P., Yue, Y., Cerretti, D. P., Dreyfus, C., and Zhou, R. (1999). Ephrin-dependent growth and pruning of hippocampal axons. Proc. Natl. Acad. Sci. U. S. A. 96, 4073–4077
Gerrow, K. and El-Husseini, A. (2006). Cell adhesion molecules at the synapse. Front. Biosci. 11, 2400–2419
Gianola, S., Savio, T., Schwab, M. E., and Rossi, F. (2003). Cell-autonomous mechanisms and myelin-associated factors contribute to the development of Purkinje axon intracortical plexus in the rat cerebellum. J. Neurosci. 23, 4613–4624
Goda, Y. and Davis, G. W. (2003). Mechanisms of synapse assembly and disassembly. Neuron 40, 243–264
Gormezano, I., Schneiderman, N., Deaux, E., and Fuentes, I. (1962). Nictitating membrane: classical conditioning and extinction in the albino rabbit. Science 138, 33–34
Grandpre, T., Nakamura, F., Vartanian, T., and Strittmatter, S. M. (2000). Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403, 439–444
Grunwald, I. C., Korte, M., Adelmann, G., Plueck, A., Kullander, K., Adams, R. H., Frotscher, M., Bonhoeffer, T., and Klein, R. (2004). Hippocampal plasticity requires postsynaptic ephrinBs. Nat. Neurosci. 7, 33–40
Grusser-Cornehls, U. and Baurle, J. (2001). Mutant mice as a model for cerebellar ataxia. Prog. Neurobiol. 63, 489–540
Hogan, A. and Faust, E. A. (1986). Nonhomologous recombination in the parvovirus chromosome: role for a CTATTTCT motif. Mol. Cell Biol. 6, 3005–3009
Huber, A. B., Weinmann, O., Brosamle, C., Oertle, T., and Schwab, M. E. (2002). Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 22, 3553–3567
Hunt, D., Coffin, R. S., Prinjha, R. K., Campbell, G., and Anderson, P. N. (2003). Nogo-A expression in the intact and injured nervous system. Mol. Cell Neurosci. 24, 1083–1102
Hunt, D., Mason, M. R., Campbell, G., Coffin, R., and Anderson, P. N. (2002). Nogo receptor mRNA expression in intact and regenerating CNS neurons. Mol. Cell Neurosci. 20, 537–552
Ito, M. (1984). The Cerebellum and Neural Control. New York: Raven Press
Josephson, A., Widenfalk, J., Widmer, H. W., Olson, L., and Spenger, C. (2001). NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp. Neurol. 169, 319–328
Kim, J. J. and Thompson, R. F. (1997). Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 20, 177–181
Koekkoek, S. K., Den Ouden, W. L., Perry, G., Highstein, S. M., and De Zeeuw, C. I. (2002). Monitoring kinetic and frequency-domain properties of eyelid responses in mice with magnetic distance measurement technique. J. Neurophysiol. 88, 2124–2133
Lewis, J., Mcgowan, E., Rockwood, J., Melrose, H., Nacharaju, P., Van Slegtenhorst, M., Gwinn-Hardy, K., Paul Murphy, M., Baker, M., Yu, X., et al. (2000). Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25, 402–405
Liu, Y. Y., Jin, W. L., Liu, H. L., and Ju, G. (2003). Electron microscopic localization of Nogo-A at the postsynaptic active zone of the rat. Neurosci. Lett. 346, 153–156
Llinas, R. and Welsh, J. P. (1993). On the cerebellum and motor learning. Curr. Opin. Neurobiol. 3, 958–965
Luo, L. (2002). Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 18, 601–635
Mangiarini, L., Sathasivam, K., Seller, M., Cozens, B., Harper, A., Hetherington, C., Lawton, M., Trottier, Y., Lehrach, H., Davies, S. W., et al. (1996). Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506
Murai, K. K., Nguyen, L. N., Irie, F., Yamaguchi, Y., and Pasquale, E. B. (2003). Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat. Neurosci. 6, 153–160
Niederost, B., Oertle, T., Fritsche, J., Mckinney, R. A., and Bandtlow, C. E. (2002). Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 22, 10368–10376
Oberdick, J., Smeyne, R. J., Mann, J. R., Zackson, S., and Morgan, J. I. (1990). A promoter that drives transgene expression in cerebellar Purkinje and retinal bipolar neurons. Science 248, 223–226
Oertle, T., Van Der Haar, M. E., Bandtlow, C. E., Robeva, A., Burfeind, P., Buss, A., Huber, A. B., Simonen, M., Schnell, L., Brosamle, C., et al. (2003). Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 23, 5393–5406
Pielage, J., Fetter, R. D., and Davis, G. W. (2005). Presynaptic spectrin is essential for synapse stabilization. Curr. Biol. 15, 918–928
Prinjha, R., Moore, S. E., Vinson, M., Blake, S., Morrow, R., Christie, G., Michalovich, D., Simmons, D. L., and Walsh, F. S. (2000). Inhibitor of neurite outgrowth in humans. Nature 403, 383–384
Salinas, P. C. and Price, S. R. (2005). Cadherins and catenins in synapse development. Curr. Opin. Neurobiol. 15, 73–80
Sastry, B. R., Morishita, W., Yip, S., and Shew, T. (1997). GABA-ergic transmission in deep cerebellar nuclei. Prog. Neurobiol. 53, 259–271
Schwab, M. E. (2004). Nogo and axon regeneration. Curr. Opin. Neurobiol. 14, 118–124
Schwab, M. E. and Caroni, P. (1988). Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J. Neurosci. 8, 2381–2393
Sereda, M. W., Meyer Zu Hörste, G., Suter, U., Uzma, N., and Nave, K. A. (2003). Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A). Nat. Med. 9, 1533–1537
Simonen, M., Pedersen, V., Weinmann, O., Schnell, L., Buss, A., Ledermann, B., Christ, F., Sansig, G., Van Der Putten, H., and Schwab, M. E. (2003). Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38, 201–211
Smeyne, R. J., Oberdick, J., Schilling, K., Berrebi, A. S., Mugnaini, E., and Morgan, J. I. (1991). Dynamic organization of developing Purkinje cells revealed by transgene expression. Science 254, 719–721
Spillmann, A. A., Bandtlow, C. E., Lottspeich, F., Keller, F., and Schwab, M. E. (1998). Identification and characterization of a bovine neurite growth inhibitor (bNI-220). J. Biol. Chem. 273, 19283–19293
Takeichi, M. and Abe, K. (2005). Synaptic contact dynamics controlled by cadherin and carenins. Trends Cell Biol. 4, 216–221
Tomomura, M., Rice, D. S., Morgan, J. I., and Yuzaki, M. (2001). Purification of Purkinje cells by fluorescence-activated cell sorting from transgenic mice that express green fluorescent protein. Eur. J. Neurosci. 14, 57–63
Wang, X., Chun, S. J., Treloar, H., Vartanian, T., Greer, C. A., and Strittmatter, S. M. (2002). Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J. Neurosci. 22, 5505–5515
Washbourne, P., Dityatev, A., Scheiffele, P., Biederer, T., Weiner, J. A., Christopherson, K. S., and El-Husseini, A. (2004). Cell adhesion molecules in synapse formation. J. Neurosci. 24, 9244–9249
Yamamoto, A., Lucas, J. J., and Hen, R. (2000). Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell 101, 57–66
Acknowledgments
We are grateful to A. McKinney and S. Hugel for help with imaging and to J. M. Fritschy, P. Scheiffele and J. P. Loeffler for kindly providing us antibodies. We thank L. Dimou for help with animal breeding, F. Christ for excellent technical support and R. Schöb and E. Hochreutener for help with the figures. This study was supported by grants of the Swiss National Science Foundation (31-63633.00), the NCCR “Neural Plasticity and Repair” of the Swiss National Science Foundation, the Spinal Cord Consortium of the Christopher Reeve Foundation (Springfield, NJ, USA), the DFG-SNF Transregio Sonderforschungsbereich Konstanz-Zurich and the EU-NeuroNetwork Project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below are the electronic supplementary materials.
Fig9
Specificity of the rabbit antiserum “Laura” (173b) antibody for Nogo-A. Immunoblotting of PC12 cells with crude antiserum (AS) and IgG purified Laura antibody shows a single band corresponding to Nogo-A. Note the specificity of the antibody and the absence of unspecific bands (JPG 95 kb)
Fig10
Nogo-A deletion does not affect the maintenance of the Purkinje cell – Deep Cerebellar Nuclei synapse. A: The mean density (± SEM) of overall Synaptophysin staining of DCN sections is similar in Nogo-A knockout and wildtype control mice. B: The number of GAD-65 positive Purkinje boutons is not significantly changed in mice lacking Nogo-A. C-F: Threshold confocal pictures of Purkinje boutons labeled for GAD-65 from wildtype (C-D) and Nogo-A knockout (E-F) mice were used for quantification of the number of Purkinje cell boutons per 100µm of DCN cell body membrane. Scale bar: 10µm (JPG 704 kb)
Fig11
Worsening of ataxia / clasping response in 14 months old L7-Nogo-A transgenic mice. Nogo-A overexpression in Purkinje neurons induces a progressive reflex reversal resulting in limb flexion instead of extension (clasping reflex) in the tail suspension test. A: at 14 months the failure of limb extension was even more severe than at 10 months (** p < 0.01, *** p < 0.001). B: correlation between Nogo-A protein expression level shown by immunofluoresence and clasping score in individual mice demonstrates that the defects displayed in the clasping reflex are positively correlated to the level of Nogo-A protein expression (** p < 0.01, *** p < 0.001) (JPG 158 kb)
Rights and permissions
About this article
Cite this article
Aloy, E.M., Weinmann, O., Pot, C. et al. Synaptic destabilization by neuronal Nogo-A. Brain Cell Bio 35, 137–157 (2006). https://doi.org/10.1007/s11068-007-9014-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11068-007-9014-3